油黄AB
油黄AB是一种偶氮类有机化合物,化学名为1-苯基偶氮基-2-萘胺,分子式C16H13N3。它被国际癌症研究机构归类为3类致癌物(尚不确定是否对人体致癌)。[1]它可由苯胺经重氮化后再与2-萘胺反应制得。[2]它和二乙酸碘苯反应,可以得到2-苯基-2H-萘并[1,2-d]三唑。[3]
| 油黄AB | |
|---|---|
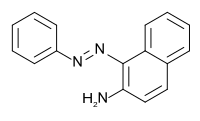 | |
| 别名 | A.F.黄2号 C.I. 11380 C.I. 食品黄10 日本黄404 油黄A 溶剂黄5 黄AB |
| 识别 | |
| CAS号 | 85-84-7 |
| SMILES |
|
| 性质 | |
| 化学式 | C16H13N3 |
| 摩尔质量 | 247.29 g·mol−1 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- . International Agency for Research on Cancer, World Health Organization. 2023-12-01 [2023-12-06]. (原始内容存档于2021-04-05).
- Journal of Physical Organic Chemistry. 2016. doi:10.1002/poc.3609
- Dyall, L., Harvey, J., & Jarman, T. (1992). Oxidative Cyclizations. VIII. Mechanisms of Oxidation ofortho-Substituted Benzenamines and Improved Cyclizations by Bis(acetato-O)Phenyliodine. Australian Journal of Chemistry, 45(2), 371. doi:10.1071/ch9920371
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.