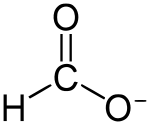
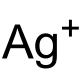甲酸银
| 甲酸银 | ||
|---|---|---|
| ||
| 识别 | ||
| CAS号 | 13126-70-0 | |
| SMILES |
| |
| 性质 | ||
| 化学式 | HCOOAg | |
| 152.887 g·mol⁻¹ | ||
| 外观 | 浅褐色固体, 光照下颜色变深[1] | |
| 熔点 | 分解 | |
| 相关物质 | ||
| 其他阴离子 | 乙酸银 苯甲酸银 | |
| 其他阳离子 | 甲酸铜 | |
| 相关化学品 | 甲酸 氧化银 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
制备
甲酸银可由甲酸和碳酸银,或甲酸盐和可溶性银盐反应,在-15~0℃反应,沉淀得到。在室温,Ag+便可和HCOO-发生氧化还原反应而析出银。[1]
- 2 HCOOH + Ag2CO3 → 2 HCOOAg + H2O + CO2↑
注释
在文献[4]中,甲酸银被标记为可溶于水。
参考文献
- George A Cowan, Fugassi James Paul. Method of making silver formate[P]. US 2630444 A. 1953.3
- A. G. Brook. Thermal Rearrangements of Organosilicon and Organogermanium Compounds[J]. J. Am. Chem. Soc., 1955. 77 (18): 4827-4829. DOI: 10.1021/ja01623a042.
- Graham A. Bowmaker, John V. Hanna, Peter C. Healy, et al. Crystal Structure, Infrared and Solid State CP MAS NMR Characterization of [(PPh3)2AgO2CH] and of [(PPh3)2AgO2CH]·2HCO2H, a Complex of the H-Bonded [H2(HCO2)3]- Species. J. Phys. Chem., 1995, 99 (12): 3909–3917. DOI: 10.1021/j100012a008.
- W. M. Haynes. CRC Handbook of Chemistry and Physics 95thed. CRC Press, 2014. pp 5-211. Solubility Chart
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.