连二亚硝酸钠
连二亚硝酸钠是一种固态的离子化合物,化学式 Na
2N
2O
2或(Na+
)2[ON=NO]2−。[1]
| 连二亚硝酸钠 | |
|---|---|
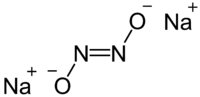 | |
| 英文名 | |
| 识别 | |
| CAS号 | (hydrate) 60884-94-8 (hydrate) |
| PubChem | 24869787 10034601, 24869787 |
| ChemSpider | 8210166 |
| SMILES |
|
| InChI |
|
| InChIKey | HLJWMCUZPYEUDI-UHFFFAOYSA-L |
| 性质 | |
| 化学式 | Na2N2O2 |
| 105.99 g·mol⁻¹ | |
| 外观 | 无色晶体 |
| 密度 | 2.466 g/cm3 |
| 熔点 | 100 °C(373 K) |
| 沸点 | 335 °C(608 K)(分解) |
| 溶解性(水) | 可溶 |
| 溶解性 | 不溶于乙醇 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
连二亚硝酸钠中的连二亚硝酸根离子 N
2O2−
2有顺反异构。反式异构体更常见,但顺式异构体也是可被制备的,反应性比反式异构体高。[1][2]
反式异构体
反式连二亚硝酸钠是无色的,可溶于水,不溶于乙醇和乙醚。[3][4]
制备
反式连二亚硝酸钠可以由亚硝酸钠被钠汞齐还原而成。[5][6][7]
- 2 NaNO2 + 4 Na(Hg) + 2 H2O → Na2N2O2 + 4 NaOH + 4 Hg
反式连二亚硝酸钠是在1927年由A. W. Scott首次合成的。它反应了亚硝酸酯、羟胺盐酸盐和乙醇钠。[4][8]
- RONO + NH2OH + 2 EtONa → Na2N2O2 + ROH + 2 EtOH
一种较早的方法由David Mendenhall于1974年发表,为一氧化氮 (NO)和金属钠在乙二醇二甲醚、甲苯和二苯基甲酮的反应。这种盐之后通过水提取。[9]
顺式异构体
参见
参考资料
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- Claus Feldmann, Martin Jansen (1996), "cis-Sodium Hyponitrite - A New Preparative Route and a Crystal Structure Analysis". Angewandte Chemie International Edition in English, volume 35, issue 15, pages 1728–1730. doi:10.1002/anie.199617281
- Trambaklal Mohanlal Oza, Rajnikant Hariprasad Thaker (1955), "The Thermal Decomposition of Silver Hyponitrite". Journal of the American Chemical society, volume 77, issue 19, pages 4976–4980. doi:10.1021/ja01624a007
- A. W. Scott (1927), "Sodium Hyponitrite". J. Am. Chem. Soc., volume = 49, issue 4, pages = 986–987. doi:10.1021/ja01403a502
- Addison, C. C.; Gamlen G. A.; Thompson, R. . J. Chem. Soc. 1952: 338. doi:10.1039/jr9520000338.
- Neumann, R. C., Jr. Bussey, R. J. . J. Am. Chem. Soc. 1970, 92 (8): 2440. doi:10.1021/ja00711a039.
- Greenwood, N. N.; Earnshaw, A. 2nd. Oxford:Butterworth-Heinemann. 1997. ISBN 0-7506-3365-4.
- Catherine E. Housecroft; Alan G. Sharpe. . 3rd. Pearson. 2008: 468. ISBN 978-0-13-175553-6.
- G. David Mendenhall (1974), "Convenient synthesis of silver hyponitrite". Journal of the American Chemical society, volume 96, issue 15, page 5000. doi:10.1021/ja00822a054
- James Riddick Partington and Chandulal Chhotalal Shah (1931), "Investigations on hyponitrites. Part I. Sodium hyponitrite: preparation and properties". Journal of the Chemical Society (Resumed), paper CCLXXXII, pages 2071-2080. doi:10.1039/JR9310002071
- C.N. Polydoropoulos, S.D. Voliotis (1967), "Sodium hyponitrite hexahydrate". Journal of Inorganic and Nuclear Chemistry, volume 29, issue 12, pages 2899–2901. doi:10.1016/0022-1902(67)80121-0
- Gary L. Stucky, Jack L. Lambert, R. Dean Dragsdorf (1969), "The hydrates of sodium hyponitrite". Journal of Inorganic and Nuclear Chemistry, volume 31, issue 1, pages 29–32 doi:10.1016/0022-1902(69)80050-3
- Charlotte N. Conner, Caroline E. Donald, Martin N. Hughes, Christina Sami (1989), "The molar absorptivity of sodium hyponitrite". Polyhedron, volume 8, issue 21, pages 2621-2622. doi:10.1016/S0277-5387(00)81166-3
- M. N. Hughes and H. G. Nicklin (1969), "The action of dinitrogen tetroxide on sodium hyponitrite". Journal of the Chemical Society D: Chemical Communications, volume 1969, issue 2, page 80a. doi:10.1039/C2969000080A
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.