雷美替胺
雷美替胺,商品名柔速瑞(Rozerem),是可治疗因难以入睡而失眠的褪黑激素受体激动剂。[2][4]它可减少入睡所需的时间,但临床益处较少。[5]它是口服药。[2]
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Rozerem, others |
| 其他名稱 | TAK-375 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605038 |
| 核准狀況 | |
| 依賴性 | 低[1] |
| 给药途径 | 口服 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 1.8%[2] |
| 血漿蛋白結合率 | 82%(大多为白蛋白)[2] |
| 药物代谢 | 肝(CYP1A2,少部分由CYP2C与CYP3A4代谢)[2] |
| 代謝產物 | M-II(活性代谢产物)[2] |
| 生物半衰期 | 雷美替胺:1–2.6小时[2] M-II:2–5小时[2][3] |
| 排泄途徑 | 尿液84%[2] 粪便4%[2] |
| 识别 | |
| |
| CAS号 | 196597-26-9 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.666 |
| 化学 | |
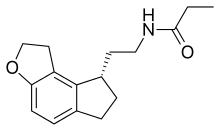
| 化学式 | C16H21NO2 |
| 摩尔质量 | 259.35 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
雷美替胺的副作用包括昏睡、头晕、疲劳、恶心、失眠加剧、激素水平改变。[2]它是褪黑激素的结构类似物,也是褪黑激素受体1A和褪黑激素受体1B的选择性激动剂。[2]雷美替胺的生物半衰期和药效持续时间都比褪黑激素长。[6]它不是苯二氮䓬类或非苯二氮䓬类药物,不作用于GABA受体,有独特的作用机制。[2][7]
参考资料
- Kim, HK; Yang, KI. . Translational and clinical pharmacology. December 2022, 30 (4): 163–171. PMC 9810491
 . PMID 36632077. doi:10.12793/tcp.2022.30.e21
. PMID 36632077. doi:10.12793/tcp.2022.30.e21  .
. - . DailyMed. 2018-12-28 [2020-04-13].
- Karim A, Tolbert D, Cao C. . Journal of Clinical Pharmacology. February 2006, 46 (2): 140–148. PMID 16432265. S2CID 38171735. doi:10.1177/0091270005283461.
- Neubauer DN. . Neuropsychiatric Disease and Treatment. February 2008, 4 (1): 69–79. PMC 2515902
 . PMID 18728808. doi:10.2147/ndt.s483
. PMID 18728808. doi:10.2147/ndt.s483  .
. - Kuriyama A, Honda M, Hayashino Y. . Sleep Medicine. April 2014, 15 (4): 385–392. PMID 24656909. doi:10.1016/j.sleep.2013.11.788.
- Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP. . Arzneimittel-Forschung. 2008, 58 (1): 1–10. PMID 18368944. S2CID 38857779. doi:10.1055/s-0031-1296459.
- Atkin T, Comai S, Gobbi G. . Pharmacological Reviews. April 2018, 70 (2): 197–245. PMID 29487083. S2CID 3578916. doi:10.1124/pr.117.014381
 .
. - Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y, et al. . Journal of Medicinal Chemistry. September 2002, 45 (19): 4222–4239. PMID 12213063. doi:10.1021/jm0201159.
- . U.S. Food and Drug Administration (FDA). 2005-10-20 [2020-04-13].
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.