 | |
| Names | |
|---|---|
| Other names
Beta-Neoendorphin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C54H77N13O12 | |
| Molar mass | 1100.289 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
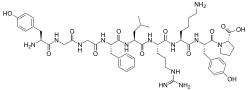
β-Neoendorphin is an endogenous opioid peptide with a nonapeptide structure and the amino acid sequence Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro (YGGFLRKYP).[1]
β-Neoendorphins (β-NEP) have the capability to stimulate wound healing by accelerating keratinocyte migration. This is achieved by β-NEP's activation of mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases 1 and 2 (ERK 1 and ERK 2); along with the upregulation of matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). Wound healing by β-NEP results in migration without consequences on proliferation in human keratinocytes.[2]
See also
References
- ↑ Minamino N.; Kangawa K.; Chino N. (1981). "β-Neo-endorphin, a new hypothalamic 'big' leu-enkephalin of porcine origin: Its purification and the complete amino acid sequence". Biochemical and Biophysical Research Communications. 99 (3): 864–870. doi:10.1016/0006-291X(81)91243-2. PMID 7247945.
- ↑ Yang, Dong Joo; Moh, Sang Hyun; Choi, Yun-Hee; Kim, Ki Woo (2020-10-12). "β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte". Molecules. 25 (20): 4640. doi:10.3390/molecules25204640. ISSN 1420-3049. PMC 7587199. PMID 33053781.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.