 | |
| Clinical data | |
|---|---|
| Trade names | Sexovid, others |
| Other names | Cyclophenil; F-6066; H-3452; ICI-48213; bis(p-Acetoxyphenyl)-cyclohexylidenemethane |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| Drug class | Selective estrogen receptor modulator; Progonadotropin |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 18–29 hours[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.264 |
| Chemical and physical data | |
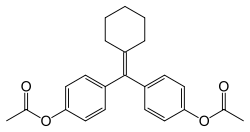
| Formula | C23H24O4 |
| Molar mass | 364.441 g·mol−1 |
| 3D model (JSmol) | |
| |
Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women.[3][4][5][6] It is mostly no longer available.[6] The medication is taken by mouth.[7][8][9]
Side effects of cyclofenil include liver toxicity among others.[10] It is a selective estrogen receptor modulator (SERM) and hence is a mixed agonist–antagonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[8] It has antiestrogenic effects on the hypothalamic–pituitary–gonadal axis and hence can increase sex hormone production and stimulate ovulation.[8][11]
Cyclofenil was introduced for medical use in 1970.[12] It has been mostly discontinued, but remains available in a few countries, including Brazil, Italy, and Japan.[6][13][3] It has been used as a doping agent by male athletes.[8]
Medical use
Cyclofenil is used to treat menstrual disturbances and anovulatory infertility caused by insufficiency of the hypothalamic–pituitary–gonadal axis in women.[3] It has also been used to treat menopausal symptoms.[3] The medication is generally used at a dosage of 400 to 600 mg per day.[3][8][9]
Available forms
Cyclofenil has been available in the form of 100, 200, and 400 mg oral tablets.[8]
Non-medical use
Cyclofenil has been used by male athletes to increase testosterone levels.[8] It is not effective for this purpose in women.[8]
Contraindications
Cyclofenil is contraindicated during pregnancy and in those with severe liver disease and unexplained uterine bleeding.[14]
Side effects
Cyclofenil is associated with a relatively high incidence of hepatotoxicity.[10] Biochemical signs of undesirable liver changes have been observed in 35% or more of individuals and 1% of individuals experience overt hepatitis.[10]
Pharmacology
Pharmacodynamics
Cyclofenil is a SERM, or a mixed agonist and antagonist of the estrogen receptors (ERs).[8] It is described as a relatively weak/mild SERM.[8] The medication is generally less effective than other SERMs.[15] The medication is an "impeded estrogen" and is thought to work as a progonadotropin by blocking the actions of estrogens in the pituitary gland and hypothalamus, thereby disinhibiting release of the gonadotropins luteinizing hormone and follicle-stimulating hormone.[11] In men, cyclofenil can increase testosterone levels due its progonadotropic effects.[8]
Pharmacokinetics
In terms of distribution, cyclofenil acts both centrally and peripherally.[15] The elimination half-life of cyclofenil after a single 200 mg dose is 18 to 29 hours.[1][2]
Chemistry
Cyclofenil is a nonsteroidal SERM and is closely related structurally to triphenylethylene SERMs like clomifene and tamoxifen.[9] It has been referred to as a diphenylethylene derivative, differing from triphenylethylenes only by the replacement of one of the phenyl rings with a cyclohexane ring.[16][11]
History
Cyclofenil was first introduced for medical use in 1970 under the brand name Ondogyne in France.[12] Subsequently, it was introduced throughout the world under a variety of other brand names, including its most well-known brand name Sexovid.[12]
Society and culture
Generic names
Cyclofenil is the English generic name of the drug and its INN, USAN, and BAN.[4][5][6]
Brand names
Cyclofenil has been marketed under a variety of brand names including Ciclifen, Fertodur, Gyneuro, Klofenil, Menoferil, Menopax, Neoclym, Oginex, Ondonid, Ondogyne, Rehibin, Sexadieno, Sexovar, and Sexovid.[17][12][13]
Availability
Cyclofenil remains available today only in Brazil, Italy, and Japan.[6][13][3] In the past, it has also been available in France, Germany, Mexico, Sweden, Switzerland, Turkey, and the United Kingdom.[5][12][13][3]
Regulation
Cyclofenil is included on the World Anti-Doping Agency list of illegal doping agents in sport.[18][19]
Research
Cyclofenil was investigated as a possible treatment for scleroderma in the 1980s, but was found to be ineffective.[20] Later study of its efficacy in treating Raynaud's phenomenon in people with scleroderma also found no significant benefit.[21]
References
- 1 2 Insler V, Lunenfeld B (January 1993). Infertility: Male and Female. Churchill Livingstone. p. 458. ISBN 978-0-443-04514-1.
- 1 2 Blankstein J, Mashiach S, Lunenfeld B (1 July 1986). Ovulation Induction and in Vitro Fertilization. Year Book Medical Publishers. p. 113. ISBN 978-0-8151-0871-9.
- 1 2 3 4 5 6 7 Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2088. ISBN 978-0-85369-840-1.
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 329–. ISBN 978-1-4757-2085-3.
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 284–. ISBN 978-3-88763-075-1.
- 1 2 3 4 5 "List of 7 Menopausal Disorders Medications Compared". Drugs.com.
- ↑ Seyffart G (6 December 2012). "Cyclofenil". Drug Dosage in Renal Insufficiency. Springer Science & Business Media. pp. 166–. ISBN 978-94-011-3804-8.
- 1 2 3 4 5 6 7 8 9 10 11 von Deutsch DA, Abukhalaf IK, Socci RR (15 October 2003). "Anabolic Doping Agents". In Mozayani A, Raymon L (eds.). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 555–. ISBN 978-1-59259-654-6.
- 1 2 3 Meniru GI, Craft IL (31 July 1997). "Ovarian stimulation for assisted reproduction technologies". In Meniru GI, Brinsden PR, Craft IL (eds.). A Handbook of Intrauterine Insemination. Cambridge University Press. pp. 58–59, 207. ISBN 978-0-521-58676-4.
- 1 2 3 Zimmerman HJ, Ishak KG (6 December 2012). "Steroids and Other Hormones". In Cameron R, Feuer G, de la Iglesia F (eds.). Drug-Induced Hepatotoxicity. Springer Science & Business Media. pp. 565–. ISBN 978-3-642-61013-4.
- 1 2 3 Bishop PM (22 October 2013). "Clinical Manifestations of Disorders of the Human Ovary". In Zuckerman S, Weir BJ (eds.). Physiology. Elsevier Science. pp. 209–. ISBN 978-1-4832-5975-8.
- 1 2 3 4 5 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 1162–. ISBN 978-0-8155-1856-3.
- 1 2 3 4 "IBM Watson Health Products". IBM Watson Health Products. Retrieved 2021-11-01.
- ↑ Mutschler E, Derendorf H, Schäfer-Korting M, Elrod K, Estes KS (1995). "Ovaries". Drug Actions: Basic Principles and Therapeutic Aspects. CRC Press. pp. 294–. ISBN 978-0-8493-7774-7.
- 1 2 Tatford EP (6 December 2012). "Excessive Vaginal Bleeding". Problems in Gynaecology. Springer Science & Business Media. pp. 105–106. ISBN 978-94-009-4125-0.
- ↑ Horsky J (6 December 2012). "Oestrogens". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 92–. ISBN 978-94-009-8195-9.
- ↑ Negwer M, Scharnow HG (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 2397. ISBN 978-3-527-30247-5.
- ↑ Chester N (13 November 2014). "Hormone and metabolic modulators". In Mottram DR, Chester N (eds.). Drugs in Sport. Routledge. pp. 117–. ISBN 978-1-134-70800-0.
- ↑ Ed The Emtree Editorial Team (1 January 2004). Doping Search Guide 2004: Over 10,000 Substance Names in Reference to the 2004 WADA (World Anti-Doping Agency) List of Prohibited Substances and Methods. Elsevier. p. 82. ISBN 978-0-444-51752-4.
- ↑ Torres MA, Furst DE (February 1990). "Treatment of generalized systemic sclerosis". Rheum Dis Clin North Am. 16 (1): 217–41. doi:10.1016/S0889-857X(21)01050-4. PMID 2406809.
- ↑ Pope J, Fenlon D, Thompson A, et al. (2000). Pope J (ed.). "Cyclofenil for Raynaud's phenomenon in progressive systemic sclerosis". Cochrane Database Syst Rev. 1998 (2): CD000955. doi:10.1002/14651858.CD000955. PMC 7032887. PMID 10796397.