 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
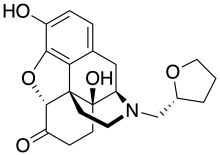
| Formula | C21H25NO5 |
| Molar mass | 371.433 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MR-2096 is an opioid analgesic drug related to oxymorphone. It has an unusual chiral tetrahydrofuran-2-ylmethyl substitution on the nitrogen which determines the character of effects, with the (R) enantiomer MR-2096 being an opioid agonist, while the (S) enantiomer MR-2097 has similarly potent opioid antagonist effects. This mix of activities has made these two enantiomers useful for characterising the binding site of the mu opioid receptor.[1]
See also
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.