
Metabolic myopathies are myopathies that result from defects in biochemical metabolism that primarily affect muscle. They are generally genetic defects (inborn errors of metabolism) that interfere with muscle's ability to create energy, causing a low ATP reservoir within the muscle cell.[1][2]
At the cellular level, metabolic myopathies lack some kind of enzyme or transport protein that prevents the chemical reactions necessary to create adenosine triphosphate (ATP).[1][3] ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. The lack of ATP prevents the muscle cells from being able to function properly. Some people with a metabolic myopathy never develop symptoms due to the body's ability to produce enough ATP through alternative pathways (e.g. the majority of those with AMP-deaminase deficiency are asymptomatic[1][4]).
H2O + ATP → H+ + ADP + Pi + energy → muscle contraction[5]
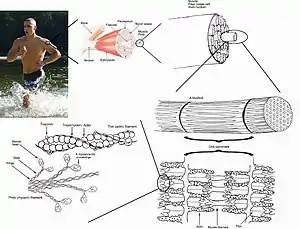
ATP is needed for muscle contraction by two processes:
- Firstly, ATP is needed for transport proteins to actively transport calcium ions into the sarcoplasmic reticulum (SR) of the muscle cell between muscle contractions. Afterwards, when a nerve signal is received, calcium channels in the SR open briefly and calcium rushes into the cytosol by selective diffusion (which does not use ATP) in what is called a "calcium spark." The diffusion of calcium ions into the cytosol causes the myosin strands of the myofibril to become exposed, and the myosin strands pull the actin microfilaments together. The muscle begins to contract.[6]
- Secondly, ATP is needed to allow the myosin to release and pull again, so that the muscle can contract further in what is known as the sliding filament model.[6]

ATP is consumed at a high rate by contracting muscles. The need for ATP in muscle cells is illustrated by the phenomenon of Rigor mortis, which is the muscle rigidity that occurs in dead bodies for a short time after death. In these muscles, all the ATP has been used up and in the absence of further ATP being generated, the calcium transport proteins stop pumping calcium ions into the sarcoplasmic reticulum and the calcium ions gradually leak out. This causes the myosin proteins to grab the actin and pull once, but without further supply of ATP, cannot release and pull again. The muscles therefore remain rigid in the position at death until the binding of myosin to actin begins to break down and they become loose again.[6]
Symptoms
In the event more ATP is needed from the affected pathway, the lack of it becomes an issue and symptoms develop. People with a metabolic myopathy often experience symptoms such as:
- exercise intolerance,
- premature muscle fatigue, pain and cramping during and/or after exercise,
- heavy breathing, shortness of breath (dyspnea), or rapid breathing (tachypnea),
- inappropriate rapid heart rate in response to exercise (tachycardia),[7][8]
- exaggerated cardiorespiratory (breath and heart rate combined) response to exercise (dyspnea/tachypnea and tachycardia),[9]
- exercise-induced myogenic hyperuricemia (exercise-induced accelerated breakdown of purine nucleotides in muscle via adenylate kinase reaction and purine nucleotide cycle),
- transient muscle contracture or pseudomyotonia (like a really bad cramp that can last for hours, which is myogenic and EMG silent),[10][8][11]
- progressive muscle weakness,
- may have a pseudoathletic appearance (hypertrophy or pseudohypertrophy) especially of the calves,[12][13][14]
- myoglobinuria and considerable breakdown of muscle tissue (rhabdomyolysis).[3][15][7]
The degree of symptoms varies greatly from person to person and is dependent on the severity of enzymatic or transport protein defect. In extreme cases it can lead to rhabdomyolysis.[16] The symptoms experienced also depend on which metabolic pathway is impaired, as different metabolic pathways produce ATP at different time periods during activity and rest, as well as the type of activity (anerobic or aerobic) and its intensity (level of ATP consumption).
A majority of patients with metabolic myopathies have dynamic rather than static findings, typically experiencing exercise intolerance, muscle pain, and cramps with exercise rather than fixed muscle weakness.[1][17] However, a minority of metabolic myopathies have fixed muscular weakness rather than exercise intolerance, imitating an inflammatory myopathy or limb girdle muscular dystrophy. It is uncommon that both static and dynamic signs predominate.[1][17]
Types
Metabolic myopathies are generally caused by an inherited genetic mutation, an inborn error of metabolism. (In livestock, an acquired environmental GSD is caused by intoxication with the alkaloid castanospermine.)[18] Metabolic myopathies cause the underproduction of adenosine triphosphate (ATP) within the muscle cell.[19]
The genetic mutation typically has an autosomal recessive hereditary pattern making it fairly rare to inherit, and even more rarely it can be caused by a random de novo genetic mutation, or autosomal dominant, X-linked, or mitochondrial.[1] Metabolic myopathies are categorized by the metabolic pathway to which the deficient enzyme or transport protein belongs. The main categories of metabolic myopathies are listed below:[20]
- Muscle glycogen storage diseases (Muscle GSDs) and other inborn errors of carbohydrate metabolism that affect muscle—defect in sugar (carbohydrate) metabolism. The deficiency occurs in the cytosol of the muscle cell.
- Fatty acid metabolism disorder (fatty acid oxidation disorder, FAOD)—defect in fat (lipid) metabolism, anywhere along the pathway, starting from entering the muscle cell and ending at converting fatty acids into acetyl-CoA within the mitochondrion. The deficiency occurs in the cell membrane, cytosol, mitochondrial membrane, or within the mitochondrion of the muscle cell.
- Nucleotide metabolism disorder—defect in purine nucleotide cycle enzyme (such as AMP deaminase deficiency).[21] Purine nucleotide metabolism is a part of protein catabolism, and the purine nucleotide cycle occurs within the cytosol of the muscle cell.
- Mitochondrial myopathy—defect in mitochondrial enzymes or transport proteins for oxidative phosphorylation (including citric acid cycle and electron transport chain), excluding those for fatty acid oxidation. Occurs in the mitochondrial membrane or within the mitochondrion of the muscle cell.
Diagnosis
The symptoms of a metabolic myopathy can be easily confused with the symptoms of another disease. As genetic sequencing research progresses, a non-invasive neuromuscular panel DNA test can help make a diagnosis. If the DNA test is inconclusive (negative or VUS), then a muscle biopsy is necessary for an accurate diagnosis.
A blood test for creatine kinase (CK) can be done under normal circumstances to test for signs of tissue breakdown, or with an added cardio portion that can indicate if muscle breakdown is occurring. In metabolic myopathies, baseline CK is either normal or elevated.[8] An electromyography (EMG) test is sometimes taken in order to rule out other disorders if the cause of fatigue is unknown.[19] In metabolic myopathies, the EMG is either normal or myopathic, but spontaneous activity is usually absent.[8]
An exercise stress test can be used to determine an inappropriate rapid heart rate (sinus tachycardia) response to exercise, which is seen in GSD-V, other glycogenoses, and mitochondrial myopathies.[7][9] A 12 Minutes Walk Test (12MWT) can also be used to determine "second wind" which is also seen in McArdle Disease (GSD-V).[7]
A cardiopulmonary exercise test can measure both heart rate and breathing, to evaluate the oxygen cost (∆V’O2/∆Work-Rate) during incremental exercise. In both glycogenoses and mitochondrial myopathies, patients displayed an increased oxygen cost during exercise compared to control subjects; and therefore, can perform less work for a given VO2 consumption during submaximal daily life exercises.[9]
In fatty acid oxidation disorders (FAOD), while at rest, some exhibit cardiac arrhythmia (commonly various forms of tachycardia, but more rarely, conduction disorders or acute bradycardia); while others have a normal heart rhythm.[22]
Some GSDs and a mitochondrial myopathy are known to have a pseudoathletic appearance. McArdle disease (GSD-V) and late-onset Pompe disease (GSD-II) are known to have hypertrophy, particularly of the calf muscles.[13][14] Cori/Forbes disease (GSD-III) is known to have hypertrophy of the sternocleidomastoid, trapezius, quadriceps, and thigh muscles.[12][23][24][25] Muscular dystrophy, limb-girdle, type 1H (which as of 2017 was excluded from LGMD for showing signs on muscle biopsy as being a mitochondrial myopathy, but not yet assigned new nomenclature)[26] is also known to have hypertrophy of the calf muscles.[27]
Differentiating between different types of metabolic myopathies can be difficult due to the similar symptoms of each type such as myoglobinuria and exercise intolerance. It has to be determined whether the patient has fixed (static) or exercise-induced (dynamic) manifestations; and if exercise-related, what kind of exercise, before extensive exercise-related lab testing is done to determine the underlying cause.[20]
Adequate knowledge is required of the body's bioenergetic systems,[8][28] including:
- which circumstances constitute anaerobic exercise (blood flow restricted by contracted muscles, insufficient oxygen and blood borne fuels, particularly isometric exercise, as well as sudden increased intensity) versus aerobic exercise (blood flow unrestricted),
- anaerobic metabolism (phosphagen system and anaerobic glycolysis - ATP produced without oxygen, regardless of adequate blood flow or not, quickly produces ATP which is useful in high-intensity activity and the beginning of any activity) versus aerobic metabolism (oxidative phosphorylation - ATP produced with oxygen, adequate blood flow required, slow to produce ATP but produces for longer and high yield),
- the different sources of ATP (phosphagen system, carbohydrate metabolism, lipid metabolism [including ketosis], protein metabolism [including the purine nucleotide cycle], oxidative phosphorylation),
- how long does each source take to start producing ATP,
- how long does each source continue to produce ATP,
- how long does each source take to replenish,
- how much ATP can each source generate,
- and which fuel source is primarily used given the intensity of the activity.
For example, leisurely-paced walking and fast-paced walking on level ground (no incline) are both aerobic, but fast-paced walking relies on more muscle glycogen because of the higher intensity (which would cause exercise intolerance symptoms in those with muscle glycogenoses that hadn't yet achieved "second wind").[10][7][15][29]
When walking at a leisurely pace on level ground (no incline), but there is loose gravel or sand, long grass, snow, mud, or walking into a headwind, that added resistance (requiring more effort) makes the activity more reliant on muscle glycogen also.[7][15] These and other surfaces, such as ice, can make you tense your muscles (which is anearobic requiring muscle glycogen) as you protect yourself from slipping or falling.[7][15]
Those with muscle glycogenoses can maintain a healthy life of exercise by learning activity adaptations, utilizing the bioenergetic systems that are available to them. Depending on the type of activity and whether they are in second wind, they slow their pace or rest briefly when need be, to make sure not to empty their "ATP Reservoir."[7][15]
| Exercise intolerance
signs and symptoms |
Triggered after prolonged activity,
and low-intensity aerobic activity |
|
Fatty acid oxidation disorder |
|
Mitochondrial myopathy | ||
| Triggered early in exercise
(within seconds to minutes), by high-intensity aerobic activity and all anaerobic activity |
|
Glycogen storage disease |
Treatment
Metabolic myopathies have varying levels of symptoms, being most severe when developed during infancy. Those who do not develop a form of a metabolic myopathy until they are in their young adult or adult life tend to have more treatable symptoms that can be helped with a change in diet and exercise.[16] It might be more accurate to say that metabolic myopathies described as adult-onset, it isn't necessarily that they didn't develop in infancy (they are inborn—from birth—errors of metabolism) but that they didn't display severe enough symptoms to warrant the attention of medical professionals until their adult years (severe symptoms such as rhabdomyolysis, fixed muscle weakness due to years of repetitive injury, or the de-conditioning of muscles from a more sedentary adult lifestyle which exacerbated symptoms).
Due to the rare nature of these diseases, it is very common to be misdiagnosed, even misdiagnosed multiple times.[10][32][28][33] Once a correct diagnosis has been made, in adult years, looking back symptoms were present since childhood, but either brushed-off as growing pains, laziness, or told that they just needed to exercise more.[28][32][10] It is especially difficult to get a diagnosis when symptoms are dynamic (exercise-induced), such as in muscle glycogenoses.[10][17][28] Sitting in a doctor's office (at rest) or doing movements that only last a few seconds (within the time limit of the phosphagen system) the patient wouldn't display any noticeable abnormalities (such as muscle fatigue, cramping, or breathlessness).
A brief or only mildly elevated heart rate (heart rate taken while sitting down after recently walking across the room or getting up on the examination table) might be assumed to be due to anxiety or illness rather than exercise-induced inappropriate rapid heart rate due to an ATP shortage in the muscle cells. In the absence of severe symptoms (such as hepatomegaly, cardiomyopathy, hypoglycemia, lactic acidosis, myoglobinuria, rhabdomyolysis, acute compartment syndrome or renal failure), it is understandable that a disease would not be noticed by medical professionals for years, when at rest the patient appears completely normal.
Depending on what enzyme is affected, a high-protein or low-fat diet may be recommended along with mild exercise. It is important for people with metabolic myopathies to consult with their doctors for a treatment plan in order to prevent acute muscle breakdowns while exercising that lead to the release of muscle proteins into the bloodstream that can cause kidney damage.[19]
A ketogenic diet has a remarkable effect on CNS-symptoms in PDH-deficiency and has also been tried in complex I deficiency.[34] A ketogenic diet has demonstrated beneficial for McArdle disease (GSD-V) as ketones readily convert to acetyl CoA for oxidative phosphorylation, whereas free fatty acids take a few minutes to convert into acetyl CoA.[31] As of 2022, another study on a ketogenic diet and McArdle disease (GSD-V) is underway.[35]
For McArdle disease (GSD-V), regular aerobic exercise utilizing "second wind" to enable the muscles to become aerobically conditioned, as well as anaerobic exercise that follows the activity adaptations so as not to cause muscle injury, helps to improve exercise intolerance symptoms and maintain overall health.[7][10][36][37] Studies have shown that regular low-moderate aerobic exercise increases peak power output, increases peak oxygen uptake (VO2peak), lowers heart rate, and lowers serum CK in individuals with McArdle disease.[36][37]
Regardless of whether the patient experiences symptoms of muscle pain, muscle fatigue, or cramping, the phenomenon of second wind having been achieved is demonstrable by the sign of an increased heart rate dropping while maintaining the same speed on the treadmill.[37][28] Inactive patients experienced second wind, demonstrated through relief of typical symptoms and the sign of an increased heart rate dropping, while performing low-moderate aerobic exercise (walking or brisk walking).[37][28] Conversely, patients that were regularly active did not experience the typical symptoms during low-moderate aerobic exercise (walking or brisk walking), but still demonstrated second wind by the sign of an increased heart rate dropping.[37][28] For the regularly active patients, it took more strenuous exercise (very brisk walking/jogging or bicycling) for them to experience both the typical symptoms and relief thereof, along with the sign of an increased heart rate dropping, demonstrating second wind.[37][28]
See also
- Bioenergetic systems
- Exercise intolerance § low ATP reservoir
- Exercise intensity § fuel used
- Myogenic hyperuricemia
- Purine nucleotide cycle § pathology (low ATP reservoir, ADP>ATP, ↑AMP)
- Tachycardia § sinus (inappropriate rapid heart rate response to exercise)
- IST § differential diagnoses (inappropriate sinus tachycardia)
- Second wind (exercise phenomenon)
- Inborn errors of carbohydrate metabolism
- Fatty acid metabolism disorder (fatty acid oxidation disorder, FAOD)
- Mitochondrial myopathies
- AMP deaminase deficiency (myoadenylate deaminase deficiency, MADD)
References
- 1 2 3 4 5 6 Urtizberea, Jon Andoni; Severa, Gianmarco; Malfatti, Edoardo (May 2023). "Metabolic Myopathies in the Era of Next-Generation Sequencing". Genes. 14 (5): 954. doi:10.3390/genes14050954. ISSN 2073-4425. PMC 10217901. PMID 37239314.
- ↑ Tobon, Alejandro (December 2013). "Metabolic myopathies". Continuum (Minneapolis, Minn.). 19 (6 Muscle Disease): 1571–1597. doi:10.1212/01.CON.0000440660.41675.06. ISSN 1538-6899. PMC 10563931. PMID 24305448.
- 1 2 "Metabolic Myopathies". www.rheumatology.org. Retrieved 2019-11-19.
- ↑ Kiani AK, Amato B, Maitz S, Nodari S, Benedetti S, Agostini F, et al. (November 2020). "Genetic test for Mendelian fatigue and muscle weakness syndromes". Acta Bio-Medica. 91 (13–S): e2020001. doi:10.23750/abm.v91i13-S.10642. PMC 8023128. PMID 33170160.
- ↑ Baker JS, McCormick MC, Robergs RA (2010). "Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise". Journal of Nutrition and Metabolism. 2010: 905612. doi:10.1155/2010/905612. PMC 3005844. PMID 21188163.
- 1 2 3 "lecture17, Energy, Use of ATP by Muscle Cells, Skeletal Muscle". www.uwyo.edu. February 18, 2005. Retrieved 2022-12-22.
- 1 2 3 4 5 6 7 8 9 10 Wakelin A (2017). Living With McArdle Disease (PDF). IAMGSD (International Association for Muscle Glycogen Disease). p. 15.
- 1 2 3 4 5 6 Bhai, S. (September 2021). "Neuromuscular Notes: Diagnosing Metabolic Myopathies". Practical Neurology. Retrieved 2023-07-30.
- 1 2 3 4 Noury, JB., Zagnoli, F., Petit, F. et al. Exercise efficiency impairment in metabolic myopathies. Sci Rep 10, 8765 (2020). doi:10.1038/s41598-020-65770-y
- 1 2 3 4 5 6 7 Lucia A, Martinuzzi A, Nogales-Gadea G, Quinlivan R, Reason S (December 2021). "Clinical practice guidelines for glycogen storage disease V & VII (McArdle disease and Tarui disease) from an international study group". Neuromuscular Disorders. 31 (12): 1296–1310. doi:10.1016/j.nmd.2021.10.006. PMID 34848128.
- ↑ Chen, Yuxi; Hagen, Michael; Lawandy, Marco; Yu, Jessi (2017-03-09). "Congenital and Acquired Myotonia". PM&R KnowledgeNow. Retrieved 2023-10-13.
- 1 2 Kishnani PS, Austin SL, Arn P, Bali DS, Boney A, Case LE, et al. (July 2010). "Glycogen storage disease type III diagnosis and management guidelines". Genetics in Medicine. 12 (7): 446–463. doi:10.1097/GIM.0b013e3181e655b6. PMID 20631546. S2CID 4609175.
- 1 2 3 Rodríguez-Gómez I, Santalla A, Díez-Bermejo J, Munguía-Izquierdo D, Alegre LM, Nogales-Gadea G, et al. (November 2018). "Non-osteogenic muscle hypertrophy in children with McArdle disease". Journal of Inherited Metabolic Disease. 41 (6): 1037–1042. doi:10.1007/s10545-018-0170-7. hdl:10578/19657. PMID 29594644. S2CID 4394513.
- 1 2 3 Menon MS, Roopch PS, Kabeer KA, Shaji CV (July 2016). "Calf Muscle Hypertrophy in Late Onset Pompe's Disease". Archives of Medicine and Health Sciences. 4 (2): 251. doi:10.4103/2321-4848.196188. ISSN 2321-4848. S2CID 58424073.
- 1 2 3 4 5 Wakelin A (2013). 101 Tips For A Good Life With McArdle Disease (PDF). AGSD-UK.
- 1 2 "Metabolic Myopathies - Signs and Symptoms". Muscular Dystrophy Association. 2015-12-18. Retrieved 2019-11-19.
- 1 2 3 Darras BT, Friedman NR (February 2000). "Metabolic myopathies: a clinical approach; part I". Pediatric Neurology. 22 (2): 87–97. doi:10.1016/S0887-8994(99)00133-2. PMID 10738913.
- ↑ Stegelmeier BL, Molyneux RJ, Elbein AD, James LF (May 1995). "The lesions of locoweed (Astragalus mollissimus), swainsonine, and castanospermine in rats". Veterinary Pathology. 32 (3): 289–98. doi:10.1177/030098589503200311. PMID 7604496. S2CID 45016726.
- 1 2 3 "Metabolic Myopathy". www.hopkinsmedicine.org. Retrieved 2019-11-19.
- 1 2 Berardo A, DiMauro S, Hirano M (March 2010). "A diagnostic algorithm for metabolic myopathies". Current Neurology and Neuroscience Reports. 10 (2): 118–126. doi:10.1007/s11910-010-0096-4. PMC 2872126. PMID 20425236.
- ↑ N.V. Bhagavan, Chung-Eun Ha, in Essentials of Medical Biochemistry (Second Edition), 2015. Nucleotide Metabolism: Myoadenylate Deaminase Deficiency
- 1 2 Bonnet D, Martin D, Villain E, Jouvet P, Rabier D, Brivet M, Saudubray JM (November 1999). "Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children". Circulation. 100 (22): 2248–2253. doi:10.1161/01.CIR.100.22.2248. PMID 10577999.
- 1 2 Marbini A, Gemignani F, Saccardi F, Rimoldi M (October 1989). "Debrancher deficiency neuromuscular disorder with pseudohypertrophy in two brothers". Journal of Neurology. 236 (7): 418–420. doi:10.1007/BF00314902. PMID 2809644. S2CID 21158814.
- 1 2 Hokezu Y, Nagamatsu K, Nakagawa M, Osame M, Ohnishi A (June 1983). "[Glycogenosis type III with peripheral nerve disorder and muscular hypertrophy in an adult]". Rinsho Shinkeigaku = Clinical Neurology. 23 (6): 473–479. PMID 6317246.
- ↑ Walters, Jon (October 2017). "Muscle hypertrophy and pseudohypertrophy". Practical Neurology. 17 (5): 369–379. doi:10.1136/practneurol-2017-001695. ISSN 1474-7766. PMID 28778933.
- 1 2 Straub V, Murphy A, Udd B (August 2018). "229th ENMC international workshop: Limb girdle muscular dystrophies - Nomenclature and reformed classification Naarden, the Netherlands, 17-19 March 2017". Neuromuscular Disorders. 28 (8): 702–710. doi:10.1016/j.nmd.2018.05.007. hdl:10138/305127. PMID 30055862. S2CID 51865029.
- 1 2 Bisceglia L, Zoccolella S, Torraco A, Piemontese MR, Dell'Aglio R, Amati A, et al. (June 2010). "A new locus on 3p23-p25 for an autosomal-dominant limb-girdle muscular dystrophy, LGMD1H". European Journal of Human Genetics. 18 (6): 636–641. doi:10.1038/ejhg.2009.235. PMC 2987336. PMID 20068593.
- 1 2 3 4 5 6 7 8 Reason SL, Voermans N, Lucia A, Vissing J, Quinlivan R, Bhai S, Wakelin A (July 2023). "Development of Continuum of Care for McArdle disease: A practical tool for clinicians and patients". Neuromuscular Disorders. 33 (7): 575–579. doi:10.1016/j.nmd.2023.05.006. PMID 37354872.
- ↑ van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ (October 2001). "The effects of increasing exercise intensity on muscle fuel utilisation in humans". The Journal of Physiology. 536 (Pt 1): 295–304. doi:10.1111/j.1469-7793.2001.00295.x. PMC 2278845. PMID 11579177.
- ↑ Scalco RS, Lucia A, Santalla A, Martinuzzi A, Vavla M, Reni G, et al. (November 2020). "Data from the European registry for patients with McArdle disease and other muscle glycogenoses (EUROMAC)". Orphanet Journal of Rare Diseases. 15 (1): 330. doi:10.1186/s13023-020-01562-x. PMC 7687836. PMID 33234167.
- 1 2 Løkken N, Hansen KK, Storgaard JH, Ørngreen MC, Quinlivan R, Vissing J. Titrating a modified ketogenic diet for patients with McArdle disease: A pilot study. J Inherit Metab Dis. 2020 Jul;43(4):778-786. doi:10.1002/jimd.12223. Epub 2020 Feb 24. PMID 32060930.
- 1 2 Reason SL (2013). One Step at a Time: Walking with McArdle Disease (PDF). AGSD-UK. ISBN 978-0-9569658-3-7.
- ↑ Scalco RS, Morrow JM, Booth S, Chatfield S, Godfrey R, Quinlivan R (September 2017). "Misdiagnosis is an important factor for diagnostic delay in McArdle disease". Neuromuscular Disorders. 27 (9): 852–855. doi:10.1016/j.nmd.2017.04.013. PMID 28629675.
- ↑ Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol. 2010;2010:340849. doi:10.1155/2010/340849. Epub 2010 May 26. PMID 20589068; PMCID: PMC2877206.
- ↑ Clinical trial number NCT04694547 for "Ketogenic Diet Survey in Patients With McArdle Disease (GSDV)" at ClinicalTrials.gov
- 1 2 Kitaoka Y (February 2014). "McArdle Disease and Exercise Physiology". Biology. 3 (1): 157–166. doi:10.3390/biology3010157. PMC 4009758. PMID 24833339.
- 1 2 3 4 5 6 Salazar-Martínez E, Santalla A, Valenzuela PL, Nogales-Gadea G, Pinós T, Morán M, et al. (2021). "The Second Wind in McArdle Patients: Fitness Matters". Frontiers in Physiology. 12: 744632. doi:10.3389/fphys.2021.744632. PMC 8555491. PMID 34721068.
Further reading
- Ch. 38. Hormonal Regulation of Energy Metabolism. Berne and Levy Physiology, 6th ed (2008)
- The effects of increasing exercise intensity on muscle fuel utilisation in humans. Van Loon et al. Journal of Physiology (2001)
- Neuromuscular Notes: Diagnosing Metabolic Myopathies. Salman Bhai, MD. Practical Neurology (2021)
External links
- Metabolic Myopathies - eMedicine
- IamGSD - International Association for Muscle Glycogen Storage Disease
- Walking With McArdle's - IamGSD videos
- EUROMAC Introduction - Video about McArdle disease and the EUROMAC Registry of McArdle disease and other rare glycogenoses patients