 | |
| Names | |
|---|---|
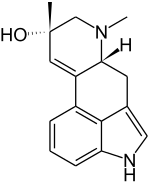
| IUPAC name
6,8β-Dimethyl-9,10-didehydroergolin-8α-ol | |
| Systematic IUPAC name
(6aR,9S)-7,9-Dimethyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinolin-9-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C16H18N2O | |
| Molar mass | 254.333 g·mol−1 |
| Appearance | prisms |
| Melting point | 229 to 234[1] °C (444 to 453 °F; 502 to 507 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Setoclavine is an ergot alkaloid.[2]
References
- ↑ J. E. Saxton (1960). "The Indole Alkaloids". In The Alkaloids, Vol. VII, (R. H. F. Manske, Ed.), pp. 4-183, New York: Academic Press.
- ↑ Liras, S; Lynch, CL; Fryer, AM; Vu, BT; Martin, SF (2001). "Applications of vinylogous Mannich reactions. Total syntheses of the Ergot alkaloids rugulovasines a and B and setoclavine". Journal of the American Chemical Society. 123 (25): 5918–24. doi:10.1021/ja010577w. PMID 11414824.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.