 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, insufflation, rectal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 20–40 min. (Oral) |
| Elimination half-life | 2.48 ± 3.20 h[1] |
| Duration of action | 4–12 hours depending on route of administration |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.088 |
| Chemical and physical data | |
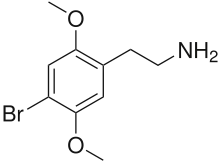
| Formula | C10H14BrNO2 |
| Molar mass | 260.131 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
2C-B (4-bromo-2,5-dimethoxyphenethylamine) is a synthetic psychedelic drug of the 2C family, initially synthesized by Alexander Shulgin in 1974 and commonly used as a recreational drug.[2] There is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant, and hallucinogen, and less commonly as an entactogen, and empathogen.[3]
2C-B is usually taken orally, but can also be insufflated or vaporized. In Shulgin's book PiHKAL, the dosage range is listed as 12–24 mg.[4]
When 2C-B is sold as a recreational drug, it is often found in a powder form, less commonly in capsules or pills.[5] It is also referred to by a number of slang names.[6]
History
2C-B was synthesized from 2,5-dimethoxybenzaldehyde by Alexander Shulgin in 1974. It first saw use among the psychiatric community as an aid during therapy. 2C-B was first sold commercially as a purported aphrodisiac[7] under the trade name "Erox", which was manufactured by the German pharmaceutical company Drittewelle.[8] For several years, it was available as tablets in Dutch smart shops under the name "Nexus" and "B-Dub".
Patterns of use
2C-B first became popularized in the United States as a short-lived substitute for the street drug ecstasy (MDMA) when the latter became illegal in 1985.[9] Many 2C-B users are young adults who attend raves.[5] Though 2C-B is still used in the rave subculture, commonly mistaken for and/or sold as Ecstasy, its intentional use has become more common in the 2000s.[10]
In 2011, street prices in the United States ranged between $10 and $30 per tablet when purchased in small quantities.[5] Larger retail purchases cost between $200 and $500 per gram. Wholesale purchases of 2C-B would lower the price ($100 to $300 per gram in 2001, $30 to $100 on the darknet in 2020).[7]
Toxicity and dosage
The September 1998 issue of Journal of Analytical Toxicology reported that very little data exists about the pharmacological properties, metabolism, and toxicity of 2C-B. The relationship between its use and death are unknown.[7] The common oral recreational dose is around 15–25 mg,[11] at which visual and auditory effects are experienced. Severe adverse reactions are extremely rare, but use of 2C-B was linked to significant brain injury in one case report; the alleged "2C-B" was never actually discovered by testing so the only evidence suggesting 2C-B was the cause was the victim's own words, without taking into consideration that adulteration and impurities are very common in illicit drugs.[12]
| Oral | Insufflated | |
|---|---|---|
| ED50 | 10 mg | 4–6 mg |
| Moderate | 15–25 mg | 5–9 mg |
| Strong | 26–35 mg | 10–20 mg |
| Extremely Intense | >35 mg | >20 mg |
| Duration | 4–8 hours | 2–4 hours |
The lethal dosage is unknown. It was reported in PiHKAL, by Alexander Shulgin, that a psychologist had accidentally taken a 100 mg dose orally without apparent harm.[4]
When sold as "Ecstasy", tablets containing 2C-B often contain about 5 mg of the drug, an amount which produces stimulatory effects that mimic the effects of MDMA; in contrast, tablets marketed as 2C-B have larger quantities of the drug (10–20 mg) which cause hallucinogenic effects.[13] Street purity of 2C-B, when tested, has been found to be relatively high.[14] Researchers in Spain found that 2C-B samples in the country doubled between 2006 and 2009, switched from primarily powder form to tablets, and exhibited "low falsification rates".[15] An analysis of street samples in the Netherlands found impurities "in small percentages"; only one of the impurities, the N-acetyl derivative of 2C-B, could be identified, and comprised 1.3% of the sample. The authors suggested that this compound was a by-product of 2C-B synthesis.[13]
Effects

Little academic research has been conducted on the effects of 2C-B in humans. The information available is largely anecdotal and limited. Effects are often described as being more easily managed than other psychedelics;[16][17] it is often compared to a mixture of a serotonergic psychedelic and MDMA.[15] At 5–10 mg, experiments with young chickens have shown it to produce effects similar to a low dosage of amphetamines.[18]
The anecdotal effects of 2C-B that have been reported by users on online discussion forums include:[19][20][21]
- At low doses, the experience may shift in intensity from engaging to mild/undetectable. Experienced users report the ability to take control of the effects and switch from engaged to sober at will.
- The hallucinations have a tendency to decrease and then increase in intensity, giving the users a sense of "waves" or even glowing. These are popularly described as "clichéd '70s visuals" or objects taking on "water color"-like textures.
- While the effects of the drug often render users unable to concentrate deeply on anything in particular, some can become engrossed in an activity such as watching a movie or playing a video game, distracting themselves from the visual and auditory effects of the drug.
- Excessive giggling or smiling is common, as is a tendency for deeper "belly laughs".
- Some users say that the effects are more intense when listening to music and report that they can see sounds and noises.
- Some users experience a decrease in visual acuity, although others report sharper vision.
- Through increased awareness of one's body, attention may be brought to perceived "imperfections" or internal body processes.
The following effects are highly dose-dependent. - Open eye visuals (OEVs), such as cartoon-like distortions and red or green halos around objects. Closed eye visuals (CEVs) are more common than OEVs.
- Affects and alters ability to communicate, engage in deep thought, or maintain attention span.
- Some users report experiencing frightening or fearful effects during the experience. Users describe feeling frigid or cold on reaching a plateau, while others feel wrapped in comfortable blankets/ultimate pleasure.
- Coordination may be affected; some users lose balance or have perceptual distinction problems.
- Onset time of 2C-B is highly dose dependent, but usually from 45 to 75 minutes. Taken on a full stomach, the onset time is increased to two hours or more.
- Before it was scheduled, 2C-B was sold in small doses as an aphrodisiac (see History). Some users report aphrodisiac effects at lower doses.[22][20]
Side effects
- Some users report mild "jitters" (body tremors), shuddering breath, and/or mild muscle spasms after insufflating 2C-B. Whether or not these effects are enjoyable depends on the user;
- Mild to intense diarrhea, gas, nausea, and general gastrointestinal discomfort;
- Severe headaches after coming down from large doses have been reported. However, many users report a lack of "comedown" or "crash", instead noting a gradual return to sobriety;
- At doses over 30–40 mg the user may experience frightening hallucinations, as well as tachycardia, hypertension, and hyperthermia;[23]
- 2C-B HCl is very painful to insufflate. Anecdotal evidence suggests that 2C-B HBr, the hydrobromide salt with greater water solubility, is less irritating to the mucous membranes lining the nose but slightly less potent when compared dose-for-dose with the HCl salt;[24]
- Rectal administration of a water-based solution of 2C-B is known to be less painful than insufflation and much more potent than oral administration.
Duration
When orally consumed, 2C-B has a much longer delay before the onset of effects than when it is insufflated. Oral ingestion generally takes roughly 45–75 minutes for the effects to be felt, plateau lasts 2–4 hours, and coming down lasts 1–2 hours. Rectal administration onset varies from 5–20 minutes. Insufflated onset takes 1–10 minutes for effects to be felt. The duration can last from 4 to 12 hours depending on route of administration, dose, and other factors.[19]
With insufflation, the effects are more abrupt and intense but have a significantly shorter duration, while oral usage results in a milder, longer experience. When insufflated, the onset happens very rapidly, usually reaching the peak at about 20–40 minutes and plateauing for 2–3 hours. 2C-B is also considered one of the most painful drugs to insufflate, with users reporting intense nasal burning.[16] The sudden intensity of the experience combined with the pain can often start the experience with a negative imprint and nausea is also increased with insufflation, compounding the issue.
Pharmacology
Unlike most psychedelics, 2C-B has been shown to be a low efficacy human serotonin 5-HT2A and 5-HT2C receptor partial agonist.[25] This suggests that activation of the 5-HT2A-coupled phospholipase D pathway[25] or functional antagonism of 5-HT2A may also play a role. The rank order of 5-HT2A receptor antagonist potency for this family of drugs in Xenopus is 2C-I > 2C-B > 2C-D > 2C-H.[26]
Research suggests that 2C-B increases dopamine levels in the brains of rats, which may contribute to its psychoactivity.[27]
Metabolism
2C-B has been shown to be metabolized by liver hepatocytes, resulting in deamination and demethylation that produces several products. Oxidative deamination results in the 2-(4-bromo-2,5-dimethoxyphenyl)-ethanol (BDMPE) and 4-bromo-2,5-dimethoxyphenylacetic acid (BDMPAA) metabolites. Additionally, 4-bromo-2,5-dimethoxybenzoic acid (BDMBA) can also be produced by oxidative deamination. Further metabolism of BDMPE and BDMPAA may occur by demethylation. Alternatively, the later metabolites can be generated by demethylation of 2C-B followed by oxidative deamination.[23]
There is species differentiation in the metabolism of 2C-B. Mice hepatocytes produce 4-bromo-2,5-dimethoxy-phenol (BDMP), a previously unknown metabolite. Meanwhile, human, monkey and rabbit hepatocytes produce 2-(4-bromo-2-hydroxy-5-methoxyphenyl)-ethanol (B-2-HMPE), but dog, rat and mouse hepatocytes do not.[23] 2C-B also reduces aggressive responses in drugged rats.[28]
Analogues and derivatives
Analogues and derivatives of 2C-B:
25-N:
- 25B-N1POMe
- 25B-NAcPip
25-NM:
- 25B-NMe7BF
- 25B-NMe7BT
- 25B-NMe7Bim
- 25B-NMe7Box
- 25B-NMe7DHBF
- 25B-NMe7Ind
- 25B-NMe7Indz
- 25B-NMePyr
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- DOB-FLY
Other:
A variety of N-substituted derivatives of 2C-B have been tested, including N-methyl-2CB, N,N-dimethyl-2CB, N-ethyl-2CB and N-benzyl-2CB. Most simple alkyl derivatives were considerably less potent than 2C-B, with N-ethyl-2CB for instance having a 40 times lower affinity for the 5-HT2A receptor. The N-benzyl derivative however was found to have higher binding affinity than 2C-B itself, with N-(4-bromobenzyl)-2CB binding even more tightly.[33] This initial research did not include functional assays of activity, but later led to the development of potent substituted N-benzyl derivatives such as 25B-NBOMe,[34] and 25B-NBOH.
Entheogenic use

2C-B was used as entheogen by the Sangoma, Nyanga, and Amagqirha people in place of their traditional plants; they refer to the chemical as Ubulawu Nomathotholo, which roughly translates to "Medicine of the Singing Ancestors".[35][36][37]
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Robadope |
|---|---|---|---|---|---|
| Yellow to green | Yellow to olive brownish | green | Yellow to black | Yellow to green | Slow pink |
| Ehrlich | Hofmann | Simon's | Scott | Folin | |
| No reaction | No reaction | No reaction | No reaction | (Light) purple | |
Drug prohibition laws
United Nations
The UN Commission on Narcotic Drugs added 2C-B to Schedule II of the Convention on Psychotropic Substances in March 2001.[38]
2C-B was mislabelled "2 C-B" in the Green List 26th edition, 2015.[39] However, this was corrected in Green List 27th edition, 2016.[40]
2C-B is a scheduled drug in most jurisdictions.[41] The following is a partial list of territories where the substance has been scheduled.
Countries
Argentina
2C-B is controlled under the List 1, as well as similar substances like 2C-I or 2C-T-2.[42]
Australia
2C-B is controlled in Australia and on the list of substances subject to import and export controls (Appendix B). It was placed on Schedule One of the Drugs Misuse and Trafficking Act when it first came to notice in 1994, when in a showcase legal battle chemist R. Simpson was charged with manufacturing the substance in Sydney. Alexander Shulgin came to Australia to testify on behalf of the defense, to no avail.
2C-B is not specifically listed in the Australia Poisons Standard (October 2015), however similar drugs such as 2C-T-2 and 2C-I are making 2C-B fall under the Australian analogue act.[43]
Belgium
In Belgium, 2C-B is a controlled substance making production, distribution, and possession illegal.
Brazil
In Brazil, 2C-B is a controlled substance making production, distribution, and possession illegal.
Canada
In Canada, 2C-B is classified under Controlled Drugs and Substances Act as Schedule III as "4-bromo-2,5-dimethoxybenzeneethanamine and any salt, isomer or salt of isomer thereof".[44]
2C-B has been rescheduled (Schedule III), in a new amendment, taking effect on October 31, 2016. This is to include the other 2C-x analogues.[45]
Chile
In August 2007, 2C-B, along with many other psychologically active substances,[46] was added to Ley 20.000, known as the Ley de Drogas.
Czech Republic
Possession of more than 200 mg of 2C-B is punishable with a two years jail sentence.[47] Smaller amount is punishable by a fine. The 200 mg threshold is merely a guideline which the court can reconsider depending on circumstances.
Denmark
Estonia
In Estonia, 2C-B is classified as Schedule I.
Germany
In Germany, 2C-B is controlled in the Betäubungsmittelgesetz (BtMG) Anlage I as "Bromdimethoxyphenethylamin" (BDMPEA).
Italy
2C-B is schedule I (tabella I).[49]
Japan
In Japan, 2C-B was scheduled in 1998. It was previously marketed as "Performax".
Luxembourg
In Luxembourg, 2C-B is a prohibited substance since 2001.[50]
Netherlands
In the Netherlands, 2C-B was scheduled on July 9, 1997.
In the Netherlands, 2C-B became a list I substance of the Opium Law despite no health incidents occurring. Following the ban, other phenethylamines were sold in place of 2C-B until the Netherlands became the first country in the world to ban 2C-I, 2C-T-2 and 2C-T-7 alongside 2C-B.
Norway
In Norway, 2C-B was classified as Schedule II on March 22, 2004, listed as 4-bromo-2,5-dimethoxyphenethylamine.[51]
Poland
2C-B is schedule I (I-P group) in Poland.
Russia
Banned as a narcotic drug with a criminal penalty for possession of at least 10 mg.[52]
Spain
In Spain, 2C-B was added to Category 2 prohibited substances in 2002.
Sweden
2C-B is currently classified as Schedule I in Sweden.
2C-B was first classified as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (Act on the Prohibition of Certain Goods Dangerous to Health) as of April 1, 1999, under SFS 1999:58[53] that made it illegal to sell or possess. Then it became schedule I as of June 1, 2002, published in LVFS 2002:4[54] but mislabeled "2-CB" in the document. However, this was corrected in a new document, LVFS 2009:22[55] effective December 9, 2009.
Switzerland
In Switzerland, 2C-B is listed in Anhang D of the DetMV and is illegal to possess.[56]
UK
All drugs in the 2C family are Class A under the Misuse of Drugs Act which means they are illegal to produce, supply or possess. Possession carries a maximum sentence of seven years imprisonment while supply is punishable by life imprisonment and an unlimited fine.[57]
United States
In the United States, 2C-B is classified as CSA Schedule I Section (d) Subsection (3) 4-Bromo-2,5-dimethoxyphenethylamine.
In the United States, a notice of proposed rulemaking published on December 20, 1994, in the Federal Register and after a review of relevant data, the Deputy Administrator of the Drug Enforcement Administration (DEA) proposed to place 4-bromo-2,5-DMPEA into Schedule I, making 2C-B illegal in the United States.[58] This became permanent law on July 2, 1995.[59]
References
- ↑ Papaseit E, Farré M, Pérez-Mañá C, Torrens M, Ventura M, Pujadas M, de la Torre R, González D (2018). "Acute Pharmacological Effects of 2C-B in Humans: An Observational Study". Frontiers in Pharmacology. 9: 206. doi:10.3389/fphar.2018.00206. PMC 5859368. PMID 29593537.
- ↑ Caudevilla-Gálligo F, Riba J, Ventura M, González D, Farré M, Barbanoj MJ, Bouso JC (July 2012). "4-Bromo-2,5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects". Journal of Psychopharmacology. 26 (7): 1026–1035. doi:10.1177/0269881111431752. PMID 22234927. S2CID 35535891.
- ↑ González D, Torrens M, Farré M (2015-10-12). "Acute Effects of the Novel Psychoactive Drug 2C-B on Emotions". BioMed Research International. 2015: 643878. doi:10.1155/2015/643878. PMC 4620274. PMID 26543863.
- 1 2 Shulgin AT (1991). Pihkal : a chemical love story. Ann Shulgin. Berkeley, CA: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- 1 2 3 "2C-B Street Names" (PDF). February 1, 2011. Archived from the original (PDF) on October 16, 2012. Retrieved 2012-09-28.
- ↑ Westhoff B (2019). Fentanyl, Inc. New York: Atlantic Monthly Press. p. 62. ISBN 978-1-0941-6390-1. OCLC 1136538402.
- 1 2 3 "2C-B (Nexus) Reappears on the Club Drug Scene" (PDF). National Drug Intelligence Center. Department of Justice. May 2001. Retrieved 11 February 2013.
- ↑ "Drittewelle 2C-B Packaging". Erowid.org. 2002. Retrieved 25 September 2013.
- ↑ Pachico E (1 November 2012). "2CB Now Drug of Choice for Colombia Elite". InSight Crime. Retrieved 11 February 2013.
- ↑ Gahlinger P (2004). Illegal Drugs: A Complete Guide to Their History, Chemistry, Use and Abuse. Penguin. pp. 343–344. ISBN 9780452285057.
- ↑ "Erowid 2C-B Vault : Dose/Dosage".
- ↑ Ambrose JB, Bennett HD, Lee HS, Josephson SA (May 2010). "Cerebral vasculopathy after 4-bromo-2,5-dimethoxyphenethylamine ingestion". The Neurologist. 16 (3): 199–202. doi:10.1097/NRL.0b013e3181a3cb53. PMID 20445431. S2CID 35035721.
- 1 2 de Boer D, Gijzels MJ, Bosman IJ, Maes RA (1999). "More data about the new psychoactive drug 2C-B". Journal of Analytical Toxicology. 23 (3): 227–8. doi:10.1093/jat/23.3.227. PMID 10369336.
- ↑ Cole MD, Lea C, Oxley N (October 2002). "4-Bromo-2,5-dimethoxyphenethylamine (2C-B): a review of the public domain literature". Science & Justice. 42 (4): 223–4. doi:10.1016/S1355-0306(02)71832-7. PMID 12632938.
- 1 2 Caudevilla-Gálligo F, Riba J, Ventura M, González D, Farré M, Barbanoj MJ, Bouso JC (July 2012). "4-Bromo-2,5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects". Journal of Psychopharmacology. 26 (7): 1026–35. doi:10.1177/0269881111431752. PMID 22234927. S2CID 35535891.
- 1 2 "Erowid 2C-B Vault: Basics". Erowid. 2011-02-20. Retrieved 2013-09-25.
- ↑ Palamar JJ, Acosta P (January 2020). "A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines". Human Psychopharmacology. 35 (1): e2719. doi:10.1002/hup.2719. PMC 6995261. PMID 31909513.
- ↑ Bronson ME, Jiang W, DeRuiter J, Clark CR (1995). "A behavioral comparison of Nexus, cathinone, BDB, and MDA". Pharmacology, Biochemistry, and Behavior. 51 (2–3): 473–475. doi:10.1016/0091-3057(95)00013-M. PMID 7667371. S2CID 32246652.
- 1 2 "Erowid 2C-B Vault: Effects". Erowid. Retrieved 2013-09-25.
- 1 2 "Drugscope: 2C-B". Drugscope. Jan 2004. Archived from the original on 2013-09-28. Retrieved 2013-09-25.
- ↑ "2C-B - Dancesafe.org". Dancesafe. Retrieved 2013-09-25.
- ↑ "Shulgin, A (1991) PIHKAL". Erowid.org. Retrieved May 15, 2012.
- 1 2 3 Carmo H, Hengstler JG, de Boer D, Ringel M, Remião F, Carvalho F, et al. (January 2005). "Metabolic pathways of 4-bromo-2,5-dimethoxyphenethylamine (2C-B): analysis of phase I metabolism with hepatocytes of six species including human". Toxicology. 206 (1): 75–89. doi:10.1016/j.tox.2004.07.004. PMID 15590110.
- ↑ "Erowid 2C-B Vault : FAQ v1.0". erowid.org.
- 1 2 Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (June 2007). "Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors". The Journal of Pharmacology and Experimental Therapeutics. 321 (3): 1054–61. CiteSeerX 10.1.1.690.3752. doi:10.1124/jpet.106.117507. PMID 17337633. S2CID 11651502.
- ↑ Villalobos CA, Bull P, Sáez P, Cassels BK, Huidobro-Toro JP (April 2004). "4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 4-HT2A receptor antagonists in Xenopus laevis oocytes". British Journal of Pharmacology. 141 (7): 1167–74. doi:10.1038/sj.bjp.0705722. PMC 1574890. PMID 15006903.
- ↑ Páleníček T, Fujáková M, et al. (January 2013). "Behavioral, neurochemical and pharmaco-EEG profiles of the psychedelic drug 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in rats". Psychopharmacology. 225 (1): 75–93. doi:10.1007/s00213-012-2797-7. PMID 22842791. S2CID 10771354.
- ↑ Muehlenkamp F, Lucion A, Vogel WH (April 1995). "Effects of selective serotonergic agonists on aggressive behavior in rats". Pharmacology Biochemistry and Behavior. 50 (4): 671–4. doi:10.1016/0091-3057(95)00351-7. PMID 7617717. S2CID 12774131.
- ↑ "Explore N-(2C-B)-Fentanyl | PiHKAL · info". isomerdesign.com.
- ↑ "Explore N-(2C-FLY)-Fentanyl | PiHKAL · info". isomerdesign.com.
- ↑ Glennon, Richard A.; Bondarev, Mikhail L.; Khorana, Nantaka; Young, Richard; May, Jesse A.; Hellberg, Mark R.; McLaughlin, Marsha A.; Sharif, Najam A. (November 2004). "β-Oxygenated Analogues of the 5-HT2ASerotonin Receptor Agonist 1-(4-Bromo-2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 47 (24): 6034–6041. doi:10.1021/jm040082s. ISSN 0022-2623. PMID 15537358.
- ↑ Beta-hydroxyphenylalkylamines and their use for treating glaucoma
- ↑ Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K (June 1994). "Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines". Journal of Medicinal Chemistry. 37 (13): 1929–35. doi:10.1021/jm00039a004. PMID 8027974.
- ↑ Heim R (March 19, 2004). Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts [Synthesis and pharmacology of potent 5-HT2A receptor agonists which have a partial N-2-methoxybenzyl structure: Development of a new structure-activity concept] (Thesis) (in German). Free University of Berlin. Retrieved August 1, 2014.
- ↑ "2CB chosen over traditional entheogen's by South African healers". Tacethno.com. 2008-03-27. Retrieved May 15, 2012.
- ↑ The Nexus Factor - An Introduction to 2C-B Erowid
- ↑ Ubulawu Nomathotholo Pack Photo by Erowid. © 2002 Erowid.org
- ↑ "List of psychotropic substances under international control" (PDF). Green List (23rd ed.). International Narcotics Control Board. August 2003. Archived from the original (PDF) on 2 March 2007.
- ↑ "List of Psychotropic Substances under International Control" (PDF). Green List (26th ed.). International Narcotics Control Board. 2015. Archived from the original (PDF) on 21 October 2017.
- ↑ "List of Psychotropic Substances under International Control" (PDF). Green List (27th ed.). International Narcotics Control Board. 2016. Archived from the original (PDF) on 2017-04-21. Retrieved 2017-04-20.
- ↑ "Erowid 2C-B page".
- ↑ "Last Argentina Controlled Drugs List" (PDF). Retrieved May 15, 2012.
- ↑ Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ↑ "CDSA Schedule II". Archived from the original on 2020-07-25. Retrieved 2008-06-13.
- ↑ "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Canada Gazette. Government of Canada, Public Works and Government Services Canada, Public Services and Procurement Canada, Integrated Services Branch, Canada. 2016-05-04.
- ↑ "APRUEBA REGLAMENTO DE LA LEY Nº 20.000 QUE SANCIONA EL TRÁFICO ILÍCITO DE ESTUPEFACIENTES Y SUSTANCIAS SICOTRÓPICAS Y SUSTITUYE LA LEY Nº 19.366" (PDF).
- ↑ "Erowid Psychoactive Vaults : Drug Laws : Czech Republic". erowid.org.
- ↑ "Bekendtgørelse om euforiserende stoffer" (in Danish). 2008-07-01. Retrieved 2013-10-01.
- ↑ "Italy Drug Schedule (Tabella I)". Archived from the original on 2011-06-27.
- ↑ Règlement grand-ducal du 14 décembre 2001 modifiant l'annexe du règlement grand-ducal modifié du 4 mars 1974 concernant certaines substances toxiques.
- ↑ "Norway Drug Schedule".
- ↑ "Постановление Правительства РФ от 01.10.2012 N 1002 "Об утверждении значительного, крупного и особо крупного размеров наркотических средств и психотропных веществ, а также значительного, крупного и особо крупного размеров для растений, содержащих наркотические средства или психотропные вещества, либо их частей, содержащих наркотические средства или психотропные вещества, для целей статей 228, 228.1, 229 и 229.1 Уголовного кодекса Российской Федерации" (с изменениями и дополнениями)". base.garant.ru.
- ↑ "Förordning (1999:58) om förbud mot vissa hälsofarliga varo" (in Swedish). 1999-02-25. Archived from the original on 2013-10-04. Retrieved 2013-10-01.
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 1997:12) om förteckningar över narkotika: LVFS 2002:4" (PDF) (in Swedish). Archived from the original (PDF) on 2013-10-04. Retrieved 2013-09-14.
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 1997:12) om förteckningar över narkotika: LVFS 2009:22" (PDF) (in Swedish). Archived from the original (PDF) on 2018-09-16. Retrieved 2013-09-14.
- ↑ "Verzeichnis aller betäubungsmittelhaltigen Stoffe" [Directory of all narcotics-containing substances]. Swissmedic (in German). Swiss Agency for Therapeutic Products. 2011-08-18. p. 2. Archived from the original (PDF) on 2012-03-15. Retrieved 2013-11-30.
- ↑ "BBC - Advice - 2CB". BBC. Retrieved 2013-10-01.
- ↑ "Schedules of Controlled Substances; Proposed Placement of 4-bromo-2,5-dimethoxyphenethylamine into Schedule I" (PDF). Federal Register. 59 (243): 65521. 20 December 1994. Retrieved 2013-09-26.
- ↑ "Erowid 2C-B Vault : DEA Ruling on Scheduling". erowid.org. Retrieved 2021-07-25.
External links
- 2C-B Entry in PiHKAL
- 2C-B Entry in PiHKAL • info
- Erowid 2C-B vault—includes reports from users of 2C-B, as well as scientific and government reports
- 2C-B Dosage chart