 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| Chemical and physical data | |
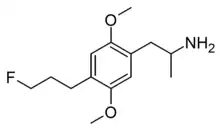
| Formula | C14H22FNO2 |
| Molar mass | 255.333 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
DOPF is a designer drug from the substituted amphetamine family. It was first synthesised by Alexander Shulgin and David Nichols in 1989 but was never published at the time, and was finally disclosed in Daniel Trachsel's review of the field in 2013. It has a binding affinity (Ki) of 9 nM at the serotonin receptor 5-HT2A but is not known to have been tested in humans.[1]
See also
References
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.