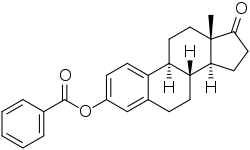
 | |
| Clinical data | |
|---|---|
| Other names | Ketohydroxyestrin benzoate; Estra-1,3,5(10)-trien-3-ol-17-one 3-benzoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H26O3 |
| Molar mass | 374.480 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estrone benzoate, or estrone 3-benzoate, is a synthetic estrogen and estrogen ester – specifically, the C3 benzoate ester of estrone – which was first reported in 1932 and was never marketed.[1][2][3][4] It led to the development in 1933 of the more active estradiol benzoate, the first estradiol ester to be introduced for medical use.[5][6][7][8]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 900–. ISBN 978-1-4757-2085-3.
- ↑ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 512–. ISBN 978-3-642-96158-8.
- ↑ Parkes AS (February 1938). "Effective Absorption of Hormones". British Medical Journal. 1 (4024): 371–373. doi:10.1136/bmj.1.4024.371. PMC 2085798. PMID 20781252.
- ↑ Butenandt A, Störmer I (1932). "Über isomere Follikelhormone. Untersuchungen über das weibliche Sexualhormon, 7. Mitteilung" [About isomeric follicle hormones. Studies on the female sex hormone, 7th communication.]. Hoppe-Seyler's Zeitschrift für physiologische Chemie. 208 (4): 129–148. doi:10.1515/bchm2.1932.208.4.129. ISSN 0018-4888.
- ↑ Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 8–. ISBN 978-3-642-58616-3.
- ↑ Miescher K, Scholz C, Tschopp E (August 1938). "The activation of female sex hormones: Mono-esters of alpha-oestradiol". The Biochemical Journal. 32 (8): 1273–1280. doi:10.1042/bj0321273b. PMC 1264184. PMID 16746750.
- ↑ Raviña E, Kubinyi H (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- ↑ Folley SJ (December 1936). "The effect of oestrogenic hormones on lactation and on the phosphatase of the blood and milk of the lactating cow". The Biochemical Journal. 30 (12): 2262–2272. doi:10.1042/bj0302262. PMC 1263335. PMID 16746289.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.