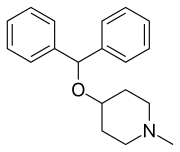
 | |
| Clinical data | |
|---|---|
| Other names | 4-(diphenylmethoxy)-1-methyl-piperidine |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 24–40 hours[1] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.170 |
| Chemical and physical data | |
| Formula | C19H23NO |
| Molar mass | 281.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Diphenylpyraline (DPP; sold as Allergen, Arbid, Belfene, Diafen, Hispril, Histyn, Lergobine, Lyssipol, Mepiben, Neargal) is a first-generation antihistamine with anticholinergic effects of the diphenylpiperidine class.[2][3][4] It is marketed in Europe for the treatment of allergies.[2][3][5] DPP has also been found to act as a dopamine reuptake inhibitor and produces hyperactivity in rodents.[6] It has been shown to be useful in the treatment of Parkinsonism.[7]
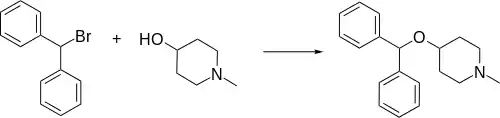
Synthesis
References
- ↑ Graham G, Bolt AG (June 1974). "Half-life of diphenylpyraline in man". Journal of Pharmacokinetics and Biopharmaceutics. 2 (3): 191–5. doi:10.1007/BF01059761. PMID 4156058. S2CID 38955052.
- 1 2 Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- 1 2 Puhakka H, Rantanen T, Virolainen E (1977). "Diphenylpyraline (Lergobine) in the treatment of patients suffering from allergic and vasomotor rhinitis". J Int Med Res. 5 (1): 37–41. doi:10.1177/030006057700500106. PMID 14039. S2CID 19330175.
- ↑ Kubo N, Shirakawa O, Kuno T, Tanaka C (March 1987). "Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay". Japanese Journal of Pharmacology. 43 (3): 277–82. doi:10.1254/jjp.43.277. PMID 2884340.
- ↑ Hruby VJ, Vardanyan R, Vardanyan R (2006). "Antihistamine Drugs". Synthesis of Essential Drugs. Amsterdam: Elsevier. ISBN 0-444-52166-6.
- ↑ Lapa G, Mathews T, Harp J, Budygin E, Jones S (2005). "Diphenylpyraline, a histamine H1 receptor antagonist, has psychostimulant properties". Eur J Pharmacol. 506 (3): 237–40. doi:10.1016/j.ejphar.2004.11.017. PMID 15627433.
- ↑ Ohno T, Kobayashi S, Hayashi M, Sakurai M, Kanazawa I (2001). "Diphenylpyraline-responsive parkinsonism in cerebrotendinous xanthomatosis: long-term follow up of three patients". J Neurol Sci. 182 (2): 95–7. doi:10.1016/S0022-510X(00)00441-X. PMID 11137513. S2CID 35946097.
- ↑ US 2479843, Knox LH, Kapp R, "1-Alkylpiperidyl-4-benzhydryl ethers, acid salts thereof and their preparation", issued 23 August 1949, assigned to Nopco Chemical Company.
- ↑ DE 934890, Schuler WA, "Verfahren zur Herstellung von basischen Benzhydryläthern [Process for the production of basic benzhydrylethers]", issued 10 November 1955, assigned to Chemische Fabrik Promonta LLC.
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| Dopaminergics |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticholinergics | |||||||||||
| Others | |||||||||||
| |||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
| DATTooltip Dopamine transporter (DRIsTooltip Dopamine reuptake inhibitors) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NETTooltip Norepinephrine transporter (NRIsTooltip Norepinephrine reuptake inhibitors) |
| ||||||||||||||
| SERTTooltip Serotonin transporter (SRIsTooltip Serotonin reuptake inhibitors) |
| ||||||||||||||
| VMATsTooltip Vesicular monoamine transporters |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.