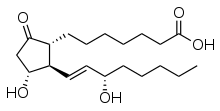
Prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids[1] having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from the fatty acid arachidonic acid.[2] Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
The structural differences between prostaglandins account for their different biological activities. A given prostaglandin may have different and even opposite effects in different tissues in some cases. The ability of the same prostaglandin to stimulate a reaction in one tissue and inhibit the same reaction in another tissue is determined by the type of receptor to which the prostaglandin binds. They act as autocrine or paracrine factors with their target cells present in the immediate vicinity of the site of their secretion. Prostaglandins differ from endocrine hormones in that they are not produced at a specific site but in many places throughout the human body.
Prostaglandins are powerful, locally-acting vasodilators and inhibit the aggregation of blood platelets. Through their role in vasodilation, prostaglandins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.[3] Conversely, thromboxanes (produced by platelet cells) are vasoconstrictors and facilitate platelet aggregation. Their name comes from their role in clot formation (thrombosis).
Specific prostaglandins are named with a letter indicating the type of ring structure, followed by a number indicating the number of double bonds in the hydrocarbon structure. For example, prostaglandin E1 has the abbreviation PGE1 and prostaglandin I2 has the abbreviation PGI2.
History and name
Systematic studies of prostaglandins began in 1930, when Kurzrock and Lieb found that human seminal fluid caused either stimulation or relaxation of strips of isolated human uterus. They noted the curious finding that uteri from patients who had gone through successful pregnancies responded to the fluid with relaxation, while uteri from sterile women responded with contraction upon addition of this seminal fluid.[4] The name prostaglandin derives from the prostate gland, chosen when prostaglandin was first isolated from seminal fluid in 1935 by the Swedish physiologist Ulf von Euler,[5] and independently by the Irish-English physiologist Maurice Walter Goldblatt (1895–1967).[6][7][8] Prostaglandins were believed to be part of the prostatic secretions, and eventually were discovered to be produced by the seminal vesicles. Later, it was shown that many other tissues secrete prostaglandins and that they perform a variety of functions. The first total syntheses of prostaglandin F2α and prostaglandin E2 were reported by E. J. Corey in 1969,[9] an achievement for which he was awarded the Japan Prize in 1989.
In 1971, it was determined that aspirin-like drugs could inhibit the synthesis of prostaglandins. The biochemists Sune K. Bergström, Bengt I. Samuelsson and John R. Vane jointly received the 1982 Nobel Prize in Physiology or Medicine for their research on prostaglandins.
Biochemistry
Biosynthesis
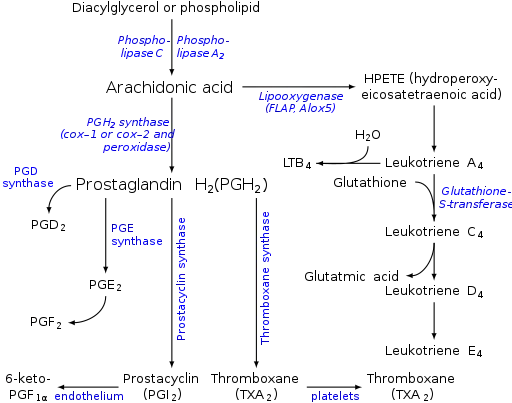
Prostaglandins are found in most tissues and organs. They are produced by almost all nucleated cells. They are autocrine and paracrine lipid mediators that act upon platelets, endothelium, uterine and mast cells. They are synthesized in the cell from the fatty acid arachidonic acid.[2]
Arachidonic acid is created from diacylglycerol via phospholipase-A2, then brought to either the cyclooxygenase pathway or the lipoxygenase pathway. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. Alternatively, the lipoxygenase enzyme pathway is active in leukocytes and in macrophages and synthesizes leukotrienes.
Release of prostaglandins from the cell
Prostaglandins were originally believed to leave the cells via passive diffusion because of their high lipophilicity. The discovery of the prostaglandin transporter (PGT, SLCO2A1), which mediates the cellular uptake of prostaglandin, demonstrated that diffusion alone cannot explain the penetration of prostaglandin through the cellular membrane. The release of prostaglandin has now also been shown to be mediated by a specific transporter, namely the multidrug resistance protein 4 (MRP4, ABCC4), a member of the ATP-binding cassette transporter superfamily. Whether MRP4 is the only transporter releasing prostaglandins from the cells is still unclear.
Cyclooxygenases
Prostaglandins are produced following the sequential oxygenation of arachidonic acid, DGLA or EPA by cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases. The classic dogma is as follows:
- COX-1 is responsible for the baseline levels of prostaglandins.
- COX-2 produces prostaglandins through stimulation.
However, while COX-1 and COX-2 are both located in the blood vessels, stomach and the kidneys, prostaglandin levels are increased by COX-2 in scenarios of inflammation and growth.
Prostaglandin E synthase
Prostaglandin E2 (PGE2) — the most abundant prostaglandin[10] — is generated from the action of prostaglandin E synthases on prostaglandin H2 (prostaglandin H2, PGH2). Several prostaglandin E synthases have been identified. To date, microsomal (named as misoprostol) prostaglandin E synthase-1 emerges as a key enzyme in the formation of PGE2.
Other terminal prostaglandin synthases
Terminal prostaglandin synthases have been identified that are responsible for the formation of other prostaglandins. For example, hematopoietic and lipocalin prostaglandin D synthases (hPGDS and lPGDS) are responsible for the formation of PGD2 from PGH2. Similarly, prostacyclin (PGI2) synthase (PGIS) converts PGH2 into PGI2. A thromboxane synthase (TxAS) has also been identified. Prostaglandin-F synthase (PGFS) catalyzes the formation of 9α,11β-PGF2α,β from PGD2 and PGF2α from PGH2 in the presence of NADPH. This enzyme has recently been crystallized in complex with PGD2[11] and bimatoprost[12] (a synthetic analogue of PGF2α).
Functions
There are currently ten known prostaglandin receptors on various cell types. Prostaglandins ligate a sub-family of cell surface seven-transmembrane receptors, G-protein-coupled receptors. These receptors are termed DP1-2, EP1-4, FP, IP1-2, and TP, corresponding to the receptor that ligates the corresponding prostaglandin (e.g., DP1-2 receptors bind to PGD2).
The diversity of receptors means that prostaglandins act on an array of cells and have a wide variety of effects such as:
- create eicosanoids hormones
- acts on thermoregulatory center of hypothalamus to produce fever
- increases mating behaviors in goldfish[13]
- Prostaglandins are released during menstruation, due to the destruction of the endometrial cells, and the resultant release of their contents.[14] Release of prostaglandins and other inflammatory mediators in the uterus cause the uterus to contract. These substances are thought to be a major factor in primary dysmenorrhea.[15][16][17]
Types
The following is a comparison of different types of prostaglandin, including prostaglandin I2 (prostacyclin; PGI2), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), and prostaglandin F2α (PGF2α).[18]
| Type | Receptor | Receptor type | Function |
|---|---|---|---|
| PGI2 | IP | Gs | |
| PGD2 | PTGDR (DP1) and CRTH2 (DP2) | GPCR |
|
| PGE2 | EP1 | Gq |
|
| EP2 | Gs |
| |
| EP3 | Gi | ||
| Unspecified | |||
| PGF2α | FP | Gq |
|
Role in pharmacology
Inhibition
Examples of prostaglandin antagonists are:
- NSAIDs (inhibit cyclooxygenase) and COX-2 selective inhibitors or coxibs
- Corticosteroids (inhibit phospholipase A2 production)
- Cyclopentenone prostaglandins may play a role in inhibiting inflammation
- Vitamin D3 and vitamin K2.[24][25][26]
Clinical uses
Synthetic prostaglandins are used:
- To induce childbirth (parturition) or abortion (PGE2 or PGF2(misoprostol), with or without mifepristone, a progesterone antagonist)
- To prevent closure of ductus arteriosus in newborns with particular cyanotic heart defects (PGE1)
- As a vasodilator in severe Raynaud syndrome or ischemia of a limb
- In pulmonary hypertension
- In treatment of glaucoma (as in bimatoprost ophthalmic solution, a synthetic prostamide analog with ocular hypotensive activity) (PGF2α)
- To treat erectile dysfunction or in penile rehabilitation following surgery (PGE1 as alprostadil).[28]
- To measure erect penis size in a clinical environment[29]
- To treat egg binding in small birds[30]
Synthesis
The original synthesis of prostaglandins F2α and E2 is shown below. It involves a Diels–Alder reaction which establishes the relative stereochemistry of three contiguous stereocenters on the prostaglandin cyclopentane core.[31]

Prostaglandin stimulants
Cold exposure and IUDs may increase prostaglandin production.[32]
See also
- Oxaprostaglandin, a type of prostaglandin
- Prostamides, a chemically related class of physiologically active substances
References
- ↑ "Eicosanoid Synthesis and Metabolism: Prostaglandins, Thromboxanes, Leukotrienes, Lipoxins". themedicalbiochemistrypage.org. Retrieved 2018-09-21.
- 1 2 Ricciotti E, FitzGerald GA (May 2011). "Prostaglandins and inflammation". Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (5): 986–1000. doi:10.1161/ATVBAHA.110.207449. PMC 3081099. PMID 21508345.
- ↑ Nelson RF (2005). An introduction to behavioral endocrinology (3rd ed.). Sunderland, Mass: Sinauer Associates. p. 100. ISBN 0-87893-617-3.
- ↑ Kurzrock, Raphael; Lieb, Charles C. (1930). "Biochemical Studies of Human Semen. II. The Action of Semen on the Human Uterus". Proceedings of the Society for Experimental Biology and Medicine. 28 (3): 268. doi:10.3181/00379727-28-5265. S2CID 85374636.
- ↑ Von Euler US (1935). "Über die spezifische blutdrucksenkende Substanz des menschlichen Prostata- und Samenblasensekrets" [On the specific blood-pressure-reducing substance of human prostate and seminal vesicle secretions]. Wiener Klinische Wochenschrift. 14 (33): 1182–1183. doi:10.1007/BF01778029. S2CID 38622866.
- ↑ Goldblatt MW (May 1935). "Properties of human seminal plasma". The Journal of Physiology. 84 (2): 208–18. doi:10.1113/jphysiol.1935.sp003269. PMC 1394818. PMID 16994667.
- ↑ Rubinstein, William D.; Jolles, Michael A.; Rubinstein, Hillary L., eds. (2011). "Goldblatt, Maurice Walter". The Palgrave Dictionary of Anglo-Jewish History. Basingstoke, England: Palgrave Macmillan. p. 333. ISBN 978-0-230-30466-6.
- ↑ R.S.F.S. (3 June 1967). "Obituary Notices: M. W. Goldblatt". British Medical Journal. 2 (5552): 644. doi:10.1136/bmj.2.5552.644. S2CID 220151673.
- ↑ Nicolaou KC, Sorensen EJ (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. p. 65. ISBN 3-527-29284-5.
- ↑ Ke J, Yang Y, Che Q, Jiang F, Wang H, Chen Z, Zhu M, Tong H, Zhang H, Yan X, Wang X, Wang F, Liu Y, Dai C, Wan X (September 2016). "Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer". Tumour Biology. 37 (9): 12203–12211. doi:10.1007/s13277-016-5087-x. PMC 5080328. PMID 27230680.
Prostaglandin E2 (PGE2) is the most abundant prostanoid in the human body
- ↑ Komoto J, Yamada T, Watanabe K, Takusagawa F (March 2004). "Crystal structure of human prostaglandin F synthase (AKR1C3)". Biochemistry. 43 (8): 2188–98. doi:10.1021/bi036046x. PMID 14979715.
- ↑ Komoto J, Yamada T, Watanabe K, Woodward DF, Takusagawa F (February 2006). "Prostaglandin F2alpha formation from prostaglandin H2 by prostaglandin F synthase (PGFS): crystal structure of PGFS containing bimatoprost". Biochemistry. 45 (7): 1987–96. doi:10.1021/bi051861t. PMID 16475787.
- ↑ "Hormonal and pheromonal control of spawning in goldfish (PDF Download Available)". ResearchGate. Retrieved 2017-02-04.
- ↑ Lethaby A, Duckitt K, Farquhar C (January 2013). "Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding". The Cochrane Database of Systematic Reviews (1): CD000400. doi:10.1002/14651858.CD000400.pub3. PMID 23440779.
- ↑ Wright, Jason and Solange Wyatt. The Washington Manual Obstetrics and Gynecology Survival Guide. Lippincott Williams & Wilkins, 2003. ISBN 0-7817-4363-X
- ↑ Harel Z (December 2006). "Dysmenorrhea in adolescents and young adults: etiology and management". Journal of Pediatric and Adolescent Gynecology. 19 (6): 363–71. doi:10.1016/j.jpag.2006.09.001. PMID 17174824.
- ↑ Bofill Rodriguez, M; Lethaby, A; Farquhar, C (19 September 2019). "Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding". The Cochrane Database of Systematic Reviews. 2019 (9): CD000400. doi:10.1002/14651858.CD000400.pub4. PMC 6751587. PMID 31535715.
- ↑ Moreno JJ (February 2017). "Eicosanoid receptors: Targets for the treatment of disrupted intestinal epithelial homeostasis". European Journal of Pharmacology. 796: 7–19. doi:10.1016/j.ejphar.2016.12.004. PMID 27940058. S2CID 1513449.
- 1 2 Rang HP (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. p. 234. ISBN 0-443-07145-4.
- ↑ Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH (March 2001). "Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation". The Journal of Clinical Investigation. 107 (5): 603–10. doi:10.1172/JCI10881. PMC 199422. PMID 11238561.
- ↑ Gross S, Tilly P, Hentsch D, Vonesch JL, Fabre JE (February 2007). "Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors". The Journal of Experimental Medicine. 204 (2): 311–20. doi:10.1084/jem.20061617. PMC 2118736. PMID 17242161.
- ↑ Stromberga, Zane; Chess-Williams, Russ; Moro, Christian (23 June 2020). "Prostaglandin E2 and F2alpha Modulate Urinary Bladder Urothelium, Lamina Propria and Detrusor Contractility via the FP Receptor". Frontiers in Physiology. 11: 705. doi:10.3389/fphys.2020.00705. PMC 7344237. PMID 32714206.
- ↑ Joshi, Shailendra; Ornstein, Eugene; Young, William L. (2010). "Cerebral and Spinal Cord Blood Flow". Cottrell and Young's Neuroanesthesia. pp. 17–59. doi:10.1016/B978-0-323-05908-4.10007-7. ISBN 978-0-323-05908-4.
- ↑ Kieronska-Rudek A, Kij A, Kaczara P, Tworzydlo A, Napiorkowski M, Sidoryk K; et al. (2021). "Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line". Cells. 10 (7): 1571. doi:10.3390/cells10071571. PMC 8303864. PMID 34206530.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Koshihara Y, Hoshi K, Shiraki M (1993). "Vitamin K2 (menatetrenone) inhibits prostaglandin synthesis in cultured human osteoblast-like periosteal cells by inhibiting prostaglandin H synthase activity". Biochem Pharmacol. 46 (8): 1355–62. doi:10.1016/0006-2952(93)90099-i. PMID 8240383.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Krishnan AV, Srinivas S, Feldman D (2009). "Inhibition of prostaglandin synthesis and actions contributes to the beneficial effects of calcitriol in prostate cancer". Dermatoendocrinol. 1 (1): 7–11. doi:10.4161/derm.1.1.7106. PMC 2715203. PMID 20046582.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "WHO Recommendations for Induction of Labour". NCBI Bookshelf. Retrieved 2020-07-15.
Induction of labour is defined as the process of artificially stimulating the uterus to start labour (1). It is usually performed by administering oxytocin or prostaglandins to the pregnant woman or by manually rupturing the amniotic membranes.
- ↑ Medscape Early Penile Rehabilitation Helps Reduce Later Intractable ED
- ↑ Veale, David; Miles, Sarah; Bramley, Sally; Muir, Gordon; Hodsoll, John (2015). "Am I normal? A systematic review and construction of nomograms for flaccid and erect penis length and circumference in up to 15 521 men". BJU International. 115 (6): 978–986. doi:10.1111/bju.13010. PMID 25487360.
- ↑ LaBonde, MS, DVM, Jerry. "Avian Reproductive and Pediatric Disorders" (PDF). Michigan Veterinary Medical Association. Archived from the original (PDF) on 2008-02-27. Retrieved 2008-01-26.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Corey, E. J.; Weinshenker, N. M.; Schaaf, T. K.; Huber, W. (1969). "Stereo-controlled synthesis of prostaglandins F-2a and E-2 (dl)". Journal of the American Chemical Society. 91 (20): 5675–7. doi:10.1021/ja01048a062. PMID 5808505.
- ↑ Mary Anne Koda-Kimble (2007). Handbook of Applied Therapeutics (8th ed.). Lippincott Williams & Wilkins. p. 1104. ISBN 978-0-7817-9026-0.
External links
- Prostaglandins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)