 | |
| Names | |
|---|---|
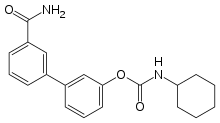
| Preferred IUPAC name
3′-Carbamoyl[1,1′-biphenyl]-3-yl cyclohexylcarbamate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.994 |
| MeSH | URB597 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H22N2O3 | |
| Molar mass | 338.407 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH).[1][2] FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.[3]
Preclinical studies have shown FAAH inhibitors to increase BDNF levels in the hippocampus and prefrontal cortex,[4] highlighting their potential in addiction treatment as "enviromimetics".[5] Indeed, Chauvet et al. found that chronic URB597 administration in rats "significantly reduces cocaine-seeking behaviour and cue- and stress-induced relapse".[6]
URB597 was at one point being developed by Kadmus Pharmaceuticals, Inc. for clinical trials in humans.[7]
See also
References
- ↑ Mor, Marco; Rivara, S; Lodola, A; Plazzi, PV; Tarzia, G; Duranti, A; Tontini, A; Piersanti, G; Kathuria, S; Piomelli, Daniele (2004). "Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies" (PDF). J Med Chem. 47 (21): 4998–5008. doi:10.1021/jm031140x. PMID 15456244. S2CID 43473180.
- ↑ Alexander, JP; Cravatt, BF (2005). "Mechanism of Carbamate Inactivation of FAAH: Implications for the Design of Covalent Inhibitors and In Vivo Functional Probes for Enzymes". Chem. Biol. 12 (11): 1179–87. doi:10.1016/j.chembiol.2005.08.011. PMC 1994809. PMID 16298297.
- ↑ Russo, R; Loverme, J; La Rana, G; Compton, TR; Parrott, J; Duranti, A; Tontini, A; Mor, M; Tarzia, G; Calignano, A.; Piomelli, D. (2007). "The fatty-acid amide hydrolase inhibitor URB597 (cyclohexylcarbamicacid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice" (PDF). J Pharmacol Exp Ther. 322 (1): 236–42. doi:10.1124/jpet.107.119941. PMID 17412883. S2CID 40603248.
- ↑ Bambico, Francis R.; Duranti, Andrea; Nobrega, José N.; Gobbi, Gabriella (March 2016). "The fatty acid amide hydrolase inhibitor URB597 modulates serotonin-dependent emotional behaviour, and serotonin1A and serotonin2A/C activity in the hippocampus". European Neuropsychopharmacology. 26 (3): 578–590. doi:10.1016/j.euroneuro.2015.12.027. hdl:11576/2631931. ISSN 1873-7862. PMID 26747370. S2CID 45109526.
- ↑ Solinas, Marcello; Chauvet, Claudia; Lafay-Chebassier, Claire; Jaafari, Nematollah; Thiriet, Nathalie (2021-02-01). "Environmental enrichment-inspired pharmacological tools for the treatment of addiction". Current Opinion in Pharmacology. 56: 22–28. doi:10.1016/j.coph.2020.09.001. ISSN 1471-4892. PMID 32966941. S2CID 221888359.
- ↑ Chauvet, Claudia; Nicolas, Céline; Thiriet, Nathalie; Lardeux, MD; Virginie; Duranti, Andrea; Solinas, Marcello (2014-12-19). "Chronic Stimulation of the Tone of Endogenous Anandamide Reduces Cue- and Stress-Induced Relapse in Rats". International Journal of Neuropsychopharmacology. 18 (1): pyu025. doi:10.1093/ijnp/pyu025. ISSN 1461-1457. PMC 4368869. PMID 25522382.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Kadmus Pharmaceuticals official website Archived December 19, 2005, at the Wayback Machine