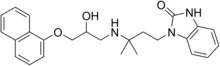 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H29N3O3 |
| Molar mass | 419.525 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Adimolol (developmental code name MEN-935) is antihypertensive agent which acts as a non-selective α1-, α2-, and β-adrenergic receptor antagonist.[1]
Synthesis
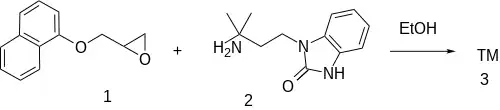
The reaction between 1-Naphthyl glycidyl ether [2461-42-9] (1) and 3-(3-amino-3-methylbutyl)-1H-benzimidazol-2-one [64928-58-1] (2) gives adimolol (3).
References
- ↑ Palluk R, Hoefke W, Gaida W, Mierau J, Bechtel WD (July 1986). "Interactions of MEN 935 (adimolol), a long acting beta- and alpha-adrenolytic antihypertensive agent, with postsynaptic alpha-adrenoceptors in different isolated blood vessels--influence of angiotensin II". Naunyn-Schmiedeberg's Archives of Pharmacology. 333 (3): 277–83. doi:10.1007/bf00512941. PMID 3020439. S2CID 24300936.
- ↑ Hoefke W, Gaida W, Palluk R, Mentrup A (1986). "Adimolol Hydrochloride Hydrate". Drugs of the Future. 11: 9. doi:10.1358/dof.1986.011.01.62036.
- ↑ US 4255430, Koppe H, Mentrup A, Renth EO, Schromm K, Hoefke W, Muacevic G, issued 1981, assigned to Boehringer Ingelheim Gmbh.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.