 | |
| Names | |
|---|---|
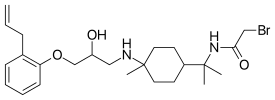
| IUPAC name
2-bromo-N-[2-[4-[[2-hydroxy-3-(2-prop-2-enylphenoxy)propyl]amino]-4-methylcyclohexyl]propan-2-yl]acetamide | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BAAM, BrAAM |
| ChEMBL | |
| ChemSpider | |
| MeSH | Bromoacetylalprenololmenthane |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H37BrN2O3 | |
| Molar mass | 481.475 g·mol−1 |
| log P | 4.14 |
| Acidity (pKa) | 13.844 |
| Basicity (pKb) | 0.153 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bromoacetylalprenololmenthane (BAAM or BrAAM) is a β adrenergic receptor agonist.[1]
References
- ↑ Rainer-Joachim; Matthys Staehelin (20 April 1987). "Bromoacetylalprenololmenthane binding to β-receptors modulates the rate of hormone-induced internalization but not desensitization in S49 cells". FEBS Letters. 214 (2): 323–326. doi:10.1016/0014-5793(87)80079-0. PMID 3032686.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.