 | |
| Names | |
|---|---|
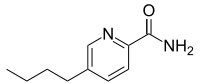
| Preferred IUPAC name
5-Butylpyridine-2-carboxamide | |
| Other names
Sch-10595; Fusaramide[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.041.024 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14N2O | |
| Molar mass | 178.235 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bupicomide is a chemical compound created and manufactured by Lanospharma Laboratories Company, Ltd. It is used experimentally as a beta blocker and clinically as a strong vasodilator with the noted side effects of reduced systolic, diastolic and mean arterial pressure.[2][3][4]
Synthesis
As the result of the screening program examining microbial fermentation products for pharmacological activity (other than antibiotic activity), fusaric acid was isolated from Fusarium oxysporum following the discovery that extracts were potent inhibitors of DBH, and thus interfered with the biosynthesis of the pressor neurohormone, norepinephrine. To refine this lead, amidation via the acid chloride was carried out to give antihypertensive analog bupicomide.[5]
References
- ↑ Bupicomide, Chemical Book
- ↑ Chrysant, SG; Adamopoulos, P; Tsuchiya, M; Frohlich, ED (1976). "Systemic and renal hemodynamic effects of bupicomide: A new vasodilator". American Heart Journal. 92 (3): 335–9. doi:10.1016/s0002-8703(76)80114-7. PMID 782220.
- ↑ Velasco, M; Gilbert, CA; Rutledge, CO; McNay, JL (1975). "Antihypertensive effect of a dopamine beta hydroxylase inhibitor, bupicomide: A comparison with hydralazine". Clinical Pharmacology and Therapeutics. 18 (2): 145–53. doi:10.1002/cpt1975182145. PMID 1097150. S2CID 19966617.
- ↑ Velasco, M.; McNay, J. L. (1977). "Physiologic mechanisms of bupicomide- and hydralazine-induced increase in plasma renin activity in hypertensive patients". Mayo Clinic Proceedings. 52 (7): 430–2. PMID 875465.
- ↑ DE 2217084, Kuniteru, Shimizu; Ushijima, Ryosuke & Sugiura, Kohsuke et al., "Verfahren zur Herstellung von 5-Alkylpyridin-derivaten [Process for the preparation of 5-alkylpyridine derivatives]", published 1972-10-19, assigned to Banyu Pharmaceutical Co. Ltd. and Hiroyoshi Hidaka
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.