 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
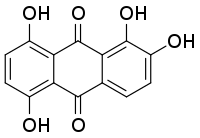
1,2,5,8-Tetrahydroxyanthracene-9,10-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.243 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H8O6 | |
| Molar mass | 272.212 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Quinalizarin or 1,2,5,8-tetrahydroxyanthraquinone is an organic compound with formula C
14H
8O
6. It is one of many tetrahydroxyanthraquinone isomers, formally derived from anthraquinone by replacement of four hydrogen atoms by hydroxyl (OH) groups at the 1, 2, 5, and 8 positions.
Quinalizarin is an inhibitor of the enzyme protein kinase CK2. It is more potent and selective than emodin.[1] It is also a potent catechol O-methyltransferase (COMT) inhibitor.[2][3]
See also
- 1,4-Dihydroxyanthraquinone (quinizarin)
- Alizarin, a related simpler dye
References
- ↑ Cozza, G.; Mazzorana, M.; Papinutto, E.; Bain, J.; Elliott, M.; di Maira, G.; Gianoncelli, A.; Pagano, M. A.; Sarno, S.; Ruzzene, M.; Battistutta, R.; Meggio, F.; Moro, S.; Zagotto, G.; Pinna, L. A. (2009). "Quinalizarin as a Potent, Selective and Cell-Permeable Inhibitor of Protein Kinase CK2" (PDF). The Biochemical Journal. 421 (3): 387–395. doi:10.1042/BJ20090069. PMID 19432557.
- ↑ Schneider J, Huh MM, Bradlow HL, Fishman J (April 1984). "Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells". J. Biol. Chem. 259 (8): 4840–5. PMID 6325410.
- ↑ Schütze N, Vollmer G, Knuppen R (April 1994). "Catecholestrogens are agonists of estrogen receptor dependent gene expression in MCF-7 cells". J. Steroid Biochem. Mol. Biol. 48 (5–6): 453–61. doi:10.1016/0960-0760(94)90193-7. PMID 8180106.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.