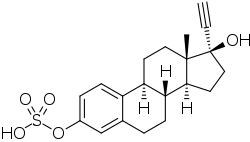
 | |
| Clinical data | |
|---|---|
| Other names | EE sulfate; 17α-Ethynylestradiol 3-sulfate |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H24O5S |
| Molar mass | 376.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ethinylestradiol sulfate (EE sulfate), also known as 17α-ethynylestradiol 3-sulfate, is an estrogen ester – specifically, the C3 sulfuric acid (sulfate) ester of the synthetic estrogen ethinylestradiol (EE) – and is the major metabolite of EE.[1][2][3] Circulating levels of EE sulfate range from 6 to 22 times those of EE when EE is taken orally.[1][2][3] EE sulfate can be transformed back into EE (14–21%) via steroid sulfatase, and it has been suggested that EE sulfate may serve as a circulating reservoir for EE, similarly to the case of estrone sulfate with estradiol.[4][5][3][1] However, the EE sulfate pool with EE is far smaller than the pool of estrone sulfate that occurs with estradiol (with estrone sulfate levels approximately 200-fold higher than estradiol levels on average with oral estradiol).[1] In addition, in contrast to the case of estrone sulfate and estrone, the conversion rate of EE sulfate back into EE is relatively low, and has been said probably isn't of clinical significance.[5] However, other studies have suggested that EE sulfate may nonetheless contribute up to 20% of total EE levels.[2][6]
See also
References
- 1 2 3 4 Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- 1 2 3 Kuhnz W, Blade H, Zimmermann H (6 December 2012). "Pharmacokinetics and Exogenous Natural and Synthetic Estrogens and Antiestrogens". In Oettel M, Schillinger E (eds.). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 284–285, 290. ISBN 978-3-642-60107-1.
- 1 2 3 Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ↑ Goldzieher JW, Mileikowsky G, Newburger J, Dorantes A, Stavchansky SA (1988). "Human pharmacokinetics of ethynyl estradiol 3-sulfate and 17-sulfate". Steroids. 51 (1–2): 63–79. doi:10.1016/0039-128x(88)90185-7. PMID 3242167. S2CID 21188869.
- 1 2 Goldzieher JW (6 December 2012). "Pharmacology of Contraceptive Steroids". In Shoupe D, Haseltine FP (eds.). Contraception. Springer Science & Business Media. pp. 19–. ISBN 978-1-4612-2730-4.
- ↑ Mattison DR, Karyakina N, Goodman M, LaKind JS (September 2014). "Pharmaco- and toxicokinetics of selected exogenous and endogenous estrogens: a review of the data and identification of knowledge gaps". Critical Reviews in Toxicology. 44 (8): 696–724. doi:10.3109/10408444.2014.930813. PMID 25099693. S2CID 11212469.