 | |
| Names | |
|---|---|
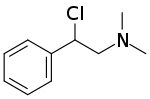
| IUPAC name
2-Chloro-N,N-dimethyl-2-phenylethanamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H14ClN | |
| Molar mass | 183.68 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
N,N-Dimethyl-2-chloro-2-phenylethylamine (DMEA) is chemical compound that irreversibly inhibits the acetylcholinesterase. DMEA can cause intoxication in cats, resulting from respiratory failure to death, and progressive damage to the central nervous system in rats.[1] Synthesis of DMEA can be obtained by treating N,N-dimethyl-2-hydroxy-2-phenylethylamine with thionyl chloride (SOCl2).[2] This compound, when dissolved water, decomposes into a highly reactive aziridinium ion, N,N-dimethyl-2-phenylaziridinium (DPA). DPA binds to the anionic site of acetylcholinesterase, where it alkylates and irreversibly inhibits the enzyme.[3][2] DMEA was also compared to N, N-dimethyl, 2-chloro-2-phenyl-1-methylethyl-amine (M-DMEA) and the results show that there is a difference between the degree of adrenergic blocking activity and their immonium ring stability in vitro. [4]
See also
References
- ↑ Ferguson, Frank C.; Wescoe, W. Clarke (1950-09-01). "The Pharmacology of N,n-Dimethyl 2-Chloro-2-Phenylethylamine". Journal of Pharmacology and Experimental Therapeutics. 100 (1): 100–114. ISSN 0022-3565. PMID 14774797.
- 1 2 Kreienkamp, HJ; Weise, C; Raba, R; Aaviksaar, A; Hucho, F (15 July 1991). "Anionic subsites of the catalytic center of acetylcholinesterase from Torpedo and from cobra venom". Proceedings of the National Academy of Sciences of the United States of America. 88 (14): 6117–21. Bibcode:1991PNAS...88.6117K. doi:10.1073/pnas.88.14.6117. PMC 52033. PMID 2068091.
- ↑ Weise, C; Kreienkamp, HJ; Raba, R; Pedak, A; Aaviksaar, A; Hucho, F (December 1990). "Anionic subsites of the acetylcholinesterase from Torpedo californica: affinity labelling with the cationic reagent N,N-dimethyl-2-phenyl-aziridinium". The EMBO Journal. 9 (12): 3885–8. doi:10.1002/j.1460-2075.1990.tb07607.x. PMC 552156. PMID 2249655.
- ↑ Ferguson, F. C. (1958-11-01). "Relation of Ethyleneimmonium Ring Stability to Adrenergic Blocking Activity in Two Haloalkylamines". Experimental Biology and Medicine. 99 (2): 362–365. doi:10.3181/00379727-99-24351. ISSN 1535-3702. PMID 13601871. S2CID 34000963.