 | |
| Clinical data | |
|---|---|
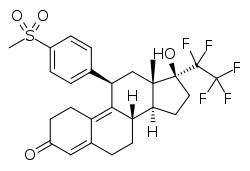
| Other names | BAY-1002670; 17β-Hydroxy-11β-[4-(methylsulfonyl)phenyl]-17α-(1,1,2,2,2-pentafluoroethyl)estra-4,9-dien-3-one |
| Routes of administration | By mouth |
| Drug class | Selective progesterone receptor modulator |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C27H29F5O4S |
| Molar mass | 544.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vilaprisan (INN, USAN) (developmental code name BAY-1002670) is a synthetic and steroidal selective progesterone receptor modulator (SPRM) which is under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis and uterine fibroids.[1][2][3] It is a potent and highly selective partial agonist of the progesterone receptor (PR).[4][2][3] As of 2017, the drug is in phase II clinical trials for the aforementioned indications.[1]
See also
References
- 1 2 "Vilaprisan - Bayer HealthCare Pharmaceuticals - AdisInsight".
- 1 2 Whitaker LH, Williams AR, Critchley HO (2014). "Selective progesterone receptor modulators". Curr. Opin. Obstet. Gynecol. 26 (4): 237–42. doi:10.1097/GCO.0000000000000082. PMID 24950125. S2CID 37474964.
- 1 2 Pluchino N, Freschi L, Wenger JM, Streuli I (2016). "Innovations in classical hormonal targets for endometriosis". Expert Rev Clin Pharmacol. 9 (2): 317–27. doi:10.1586/17512433.2016.1129895. PMID 26645363. S2CID 8624056.
- ↑ Schütt B, Kaiser A, Schultze-Mosgau MH, Seitz C, Bell D, Koch M, Rohde B (2016). "Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double-blind, randomized, placebo-controlled phase 1 trial in healthy women". Hum. Reprod. 31 (8): 1703–12. doi:10.1093/humrep/dew140. PMID 27288475.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.