 | |
 | |
| Clinical data | |
|---|---|
| Other names | 17α-Methyl-19-nor-δ9-testosterone; 17α-Methylestra-4,9-dien-17β-ol-3-one |
| Routes of administration | By mouth |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
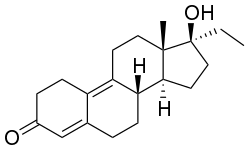
Ethyldienolone, also known as 17α-methyl-19-nor-δ9-testosterone, as well as 17α-methylestra-4,9-dien-17β-ol-3-one, is synthetic, orally active anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 19-nortestosterone. It is slightly more active than methyltestosterone when given orally.[1] Ethyldienolone is closely related to dienolone and methyldienolone.
See also
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.