 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2-Hydroxyflutamide; HF; OHF; Flutamide-hydroxide; SCH-16423; Hydroxyniphtholide; Hydroxyniftolide; α,α,α-Trifluoro-2-methyl-4'-nitro-m-lactotoluidide |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.708 |
| Chemical and physical data | |
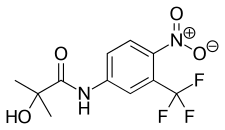
| Formula | C11H11F3N2O4 |
| Molar mass | 292.214 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Hydroxyflutamide (HF, OHF) (developmental code name SCH-16423), or 2-hydroxyflutamide, is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of flutamide, which is considered to be a prodrug of hydroxyflutamide as the active form.[1][2] It has been reported to possess an IC50 of 700 nM for the androgen receptor (AR), which is about 4-fold less than that of bicalutamide.[3]
| Compound | RBA[lower-alpha 2] |
|---|---|
| Metribolone | 100 |
| Dihydrotestosterone | 85 |
| Cyproterone acetate | 7.8 |
| Bicalutamide | 1.4 |
| Nilutamide | 0.9 |
| Hydroxyflutamide | 0.57 |
| Flutamide | <0.0057 |
Notes:
| |
| Species | IC50 (nM) | RBA (ratio) | ||||
|---|---|---|---|---|---|---|
| Bicalutamide | 2-Hydroxyflutamide | Nilutamide | Bica / 2-OH-flu | Bica / nilu | Ref | |
| Rat | 190 | 700 | ND | 4.0 | ND | [5] |
| Rat | ~400 | ~900 | ~900 | 2.3 | 2.3 | [6] |
| Rat | ND | ND | ND | 3.3 | ND | [7] |
| Rata | 3595 | 4565 | 18620 | 1.3 | 5.2 | [8] |
| Human | ~300 | ~700 | ~500 | 2.5 | 1.6 | [9] |
| Human | ~100 | ~300 | ND | ~3.0 | ND | [10] |
| Humana | 2490 | 2345 | 5300 | 1.0 | 2.1 | [8] |
| Footnotes: a = Controversial data. Sources: See template. | ||||||
| Antiandrogen | Relative potency |
|---|---|
| Bicalutamide | 4.3 |
| Hydroxyflutamide | 3.5 |
| Flutamide | 3.3 |
| Cyproterone acetate | 1.0 |
| Zanoterone | 0.4 |
| Description: Relative potencies of orally administered antiandrogens in antagonizing 0.8 to 1.0 mg/kg s.c. testosterone propionate-induced ventral prostate weight increase in castrated immature male rats. Higher values mean greater potency. Sources: See template. | |
References
- ↑ Serra C, Sandor NL, Jang H, Lee D, Toraldo G, Guarneri T, et al. (December 2013). "The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups". Endocrinology. 154 (12): 4594–4606. doi:10.1210/en.2013-1004. PMC 3836062. PMID 24105483.
- ↑ Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–247. doi:10.2174/0929867003375371. PMID 10637363.
- ↑ Furr BJ (June 1995). "Casodex: preclinical studies and controversies". Annals of the New York Academy of Sciences. 761 (3): 79–96. Bibcode:1995NYASA.761...79F. doi:10.1111/j.1749-6632.1995.tb31371.x. PMID 7625752. S2CID 37242269.
- ↑ Ayub M, Levell MJ (August 1989). "The effect of ketoconazole related imidazole drugs and antiandrogens on [3H] R 1881 binding to the prostatic androgen receptor and [3H]5 alpha-dihydrotestosterone and [3H]cortisol binding to plasma proteins". J. Steroid Biochem. 33 (2): 251–5. doi:10.1016/0022-4731(89)90301-4. PMID 2788775.
- ↑ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H (June 1987). "ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen". The Journal of Endocrinology. 113 (3): R7–R9. doi:10.1677/joe.0.113R007. PMID 3625091.
- ↑ Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, et al. (January 1994). "Non-steroidal antiandrogens: synthesis and biological profile of high-affinity ligands for the androgen receptor". The Journal of Steroid Biochemistry and Molecular Biology. 48 (1): 111–119. doi:10.1016/0960-0760(94)90257-7. PMID 8136296. S2CID 31404295.
- ↑ Winneker RC, Wagner MM, Batzold FH (December 1989). "Studies on the mechanism of action of Win 49596: a steroidal androgen receptor antagonist". Journal of Steroid Biochemistry. 33 (6): 1133–1138. doi:10.1016/0022-4731(89)90420-2. PMID 2615358.
- 1 2 Luo S, Martel C, Leblanc G, Candas B, Singh SM, Labrie C, et al. (1996). "Relative potencies of Flutamide and Casodex: preclinical studies". Endocrine Related Cancer. 3 (3): 229–241. doi:10.1677/erc.0.0030229. ISSN 1351-0088.
- ↑ Ayub M, Levell MJ (August 1989). "The effect of ketoconazole related imidazole drugs and antiandrogens on [3H] R 1881 binding to the prostatic androgen receptor and [3H]5 alpha-dihydrotestosterone and [3H]cortisol binding to plasma proteins". Journal of Steroid Biochemistry. 33 (2): 251–255. doi:10.1016/0022-4731(89)90301-4. PMID 2788775.
- ↑ Kemppainen JA, Wilson EM (July 1996). "Agonist and antagonist activities of hydroxyflutamide and Casodex relate to androgen receptor stabilization". Urology. 48 (1): 157–163. doi:10.1016/S0090-4295(96)00117-3. PMID 8693644.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.