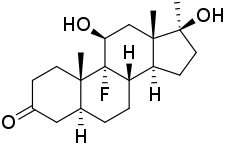
 | |
| Clinical data | |
|---|---|
| Other names | U-7265; 5α-Dihydrofluoxymesterone; 9α-Fluoro-11β-hydroxy-17α-methyl-5α-dihydrotestosterone; 9α-Fluoro-11β-hydroxy-17α-methyl-5α-DHT; 9α-Fluoro-11β-hydroxy-17α-methyl-5α-androstan-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| Chemical and physical data | |
| Formula | C20H30FO3 |
| Molar mass | 337.455 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dihydrofluoxymesterone (developmental code name U-7265) is an androgen and anabolic steroid (AAS) which was never marketed.[1][2][3] It was assessed in the treatment of breast cancer in women in at least one clinical study in the 1970s and showed effectiveness similar to that of other AAS.[1][2] The drug is the 5α-reduced analogue and metabolite of fluoxymesterone.[1][2][3]
See also
References
- 1 2 3 Gordan, G. S. (1976). "Cancer in Man". Anabolic-Androgenic Steroids. pp. 499–513. doi:10.1007/978-3-642-66353-6_16. ISBN 978-3-642-66355-0.
- 1 2 3 Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 504–. ISBN 978-3-642-66353-6.
- 1 2 Kammerer RC, Merdink JL, Jagels M, Catlin DH, Hui KK (1990). "Testing for fluoxymesterone (Halotestin) administration to man: identification of urinary metabolites by gas chromatography-mass spectrometry". J. Steroid Biochem. 36 (6): 659–66. doi:10.1016/0022-4731(90)90185-u. PMID 2214783.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.