 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Azilect, Azipron, others |
| Other names | VP-1012, N-propargyl-1(R)-aminoindan[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606017 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 36% |
| Protein binding | 88 – 94% |
| Metabolism | Liver (CYP1A2-mediated) |
| Elimination half-life | 3 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.301.709 |
| Chemical and physical data | |
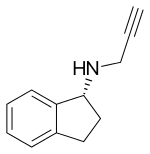
| Formula | C12H13N |
| Molar mass | 171.243 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rasagiline (Azilect, Azipron) is an irreversible inhibitor of monoamine oxidase-B[3] used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.[4]
The racemic form of the drug was invented by Aspro Nicholas in the early 1970s. Moussa B.H. Youdim identified it as a potential drug for Parkinson's disease, and working with collaborators at Technion – Israel Institute of Technology in Israel and the drug company, Teva Pharmaceuticals, identified the R-isomer as the active form of the drug.[5] Teva brought it to market in partnership with Lundbeck in Europe and Eisai in the US and elsewhere. It was approved in Europe in 2005 and in the US in 2006.
Rasagiline is used to treat symptoms of Parkinson's disease both alone and in combination with other drugs. It has shown efficacy in both early and advanced Parkinsons, and appears to be especially useful in dealing with non-motor symptoms like fatigue.[6][7][8]
Rasagiline has not been tested in pregnant women and is Pregnancy Category C in the US.[8]
Side effects
The FDA label contains warnings that rasagiline may cause severe hypertension or hypotension, may make people sleepy, may make motor control worse in some people, may cause hallucinations and psychotic-like behavior, may cause impulse control disorder, may increase the risk of melanoma, and upon withdrawal may cause high fever or confusion.[8]
Side effects when the drug is taken alone include flu-like symptoms, joint pain, depression, stomach upset, headache, dizziness, and insomnia. When taken with L-DOPA, side effects include increased movement problems, accidental injury, sudden drops in blood pressure, joint pain and swelling, dry mouth, rash, abnormal dreams and digestive problems including vomiting, loss of appetite, weight loss, abdominal pain, nausea, constipation.[8] When taken with Parkinson's drugs other than L-DOPA, side effects include peripheral edema, fall, joint pain, cough, and insomnia.[8]
Interactions
People who are taking meperidine, tramadol, methadone, propoxyphene, dextromethorphan, St. John’s wort, cyclobenzaprine, or another MAO inhibitor should not take rasagiline.[8]
The FDA drug label carries a warning of the risk of serotonin syndrome when rasagiline is used with antidepressants or with meperidine.[8] However the risk appears to be low, based on a multicenter retrospective study in 1504 people, which looked for serotonin syndrome in people with PD who were treated with rasagiline plus antidepressants, rasagiline without antidepressants, or antidepressants plus Parkinson's drugs other than either rasagiline or selegiline; no cases were identified.[6]
There is a risk of psychosis or bizarre behavior if rasagiline is used with dextromethorphan and there is a risk of non-selective MAO inhibition and hypertensive crisis if rasagiline is used with other MAO inhibitors.[8]
Chemistry
Rasagiline is molecularly a propargylamine derivative.[9] The form brought to market by Teva and its partners is the mesylate salt, and was designated chemically as: 1H-Inden-1-amine-2,3-dihydro-N-2-propynyl-(1R)-methanesulfonate.[8]
Pharmacology
Mechanism of action
Parkinson's disease is characterized by the death of cells that produce dopamine, a neurotransmitter. An enzyme called monoamine oxidase (MAO) breaks down neurotransmitters. MAO has two forms, MAO-A and MAO-B. MAO-B is generally believed to break down dopamine; however, recent evidence suggests that MAO-A may mostly or entirely be responsible for dopamine metabolism.[10] Rasagiline prevents the breakdown of dopamine by irreversibly binding to MAO-B. Dopamine is therefore more available, somewhat compensating for the diminished quantities made in the brains of people with Parkinson's.[6]
Selegiline was the first selective MAO-B inhibitor. It is partly metabolized to levomethamphetamine (l-methamphetamine), one of the two enantiomers of methamphetamine, in vivo.[11][12] While these metabolites may contribute to selegiline's ability to inhibit reuptake of the neurotransmitters dopamine and norepinephrine, they have also been associated with orthostatic hypotension and hallucinations in some people.[12][13][14] Rasagiline metabolizes into 1(R)-aminoindan which has no amphetamine-like characteristics[15] and has neuroprotective properties in cells and in animal models.[16]
It is selective for MAO type B over type A by a factor of fourteen.[17]
Metabolism
Rasagiline is broken down via CYP1A2,[18] part of the cytochrome P450 metabolic path in the liver. It is contraindicated in patients with hepatic insufficiency and its use should be monitored carefully in patients taking other drugs that alter the normal effectiveness of this metabolic path.[8]
History
Prior to the discovery of rasagiline, a closely related analog called SU-11739 (AGN 1133) was patented.[19] At first, the N-methyl was necessary for the agent to be considered a ring cyclized analog of pargyline with ca. twenty-times the potency.[20] However, the N-methyl compound was a non-selective MAOI.[21]
Racemic rasagiline was discovered and patented by Aspro Nicholas in the 1970s as a drug candidate for treatment of hypertension.[22]
Moussa B. H. Youdim, a biochemist, had been involved in developing selegiline as a drug for Parkinsons, in collaboration with Peter Reiderer. He wanted to find a similar compound that would have fewer side effects, and around 1977, at about the same time he moved from London to Haifa to join the faculty of Technion, he noticed that rasagiline could potentially be such a compound.[23] He called that compound, AGN 1135.[24]
In 1996 Youdim, in collaboration with scientists from Technion and the US National Institutes of Health, and using compounds developed with Teva Pharmaceuticals, published a paper in which the authors wrote that they were inspired by the racemic nature of deprenyl and the greater activity of one of its stereoisomers, L-deprenyl, which became selegiline, to explore the qualities of the isomers of the Aspro compound, and they found that the R-isomer had almost all the activity; this is the compound that became rasagiline.[24] They called the mesylate salt of the R-isomer TVP-1012 and the hydrochloride salt, TVP-101.[24]
Teva and Technion filed patent applications for this racemically pure compound, methods to make it, and methods to use it to treat Parkinsons and other disorders, and Technion eventually assigned its rights to Teva.[22]
Teva began development of rasagiline, and by 1999 was in Phase III trials, and entered into a partnership with Lundbeck in which Lundbeck agreed to share the costs and obtained the joint right to market the drug in Europe.[25] In 2003 Teva partnered with Eisai, giving Eisai the right to jointly market the drug for Parkinson's in the US, and to co-develop and co-market the drug for Alzheimers and other neurological diseases.[26]
It was approved by the European Medicines Agency for Parkinson's in 2005[16] and in the US in 2006.[9]: 255
Research
Rasagiline was tested for efficacy in people with multiple system atrophy in a large randomized, placebo-controlled, double-blind disease-modification trial; the drug failed.[7]
Teva conducted clinical trials attempting to prove that rasagiline did not just treat symptoms, but was a disease-modifying drug - that it actually prevented the death of the dopaminergic neurons that characterize Parkinson's disease and slowed disease progression. They conducted two clinical trials, called TEMPO and ADAGIO, to try to prove this. The FDA advisory committee rejected their claim in 2011, saying that the clinical trial results did not prove that rasagiline was neuroprotective. The main reason was that in one of the trials, the lower dose was effective at slowing progression, but the higher dose was not, and this made no sense in light of standard dose-response pharmacology.[27][28]
See also
References
- ↑ Akao Y, Maruyama W, Yi H, Shamoto-Nagai M, Youdim MB, Naoi M (June 2002). "An anti-Parkinson's disease drug, N-propargyl-1(R)-aminoindan (rasagiline), enhances expression of anti-apoptotic bcl-2 in human dopaminergic SH-SY5Y cells". Neuroscience Letters. 326 (2): 105–8. doi:10.1016/s0304-3940(02)00332-4. PMID 12057839. S2CID 29736753.
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ Oldfield V, Keating GM, Perry CM (2007). "Rasagiline: a review of its use in the management of Parkinson's disease". Drugs. 67 (12): 1725–47. doi:10.2165/00003495-200767120-00006. PMID 17683172. S2CID 195688993.
- ↑ Gallagher DA, Schrag A (2008). "Impact of newer pharmacological treatments on quality of life in patients with Parkinson's disease". CNS Drugs. 22 (7): 563–86. doi:10.2165/00023210-200822070-00003. PMID 18547126. S2CID 29707067.
- ↑ Lakhan SE (July 2007). "From a Parkinson's disease expert: Rasagiline and the future of therapy" (PDF). Molecular Neurodegeneration. 2 (1): 13. doi:10.1186/1750-1326-2-13. PMC 1929084. PMID 17617893.
- 1 2 3 Stocchi F, Fossati C, Torti M (2015). "Rasagiline for the treatment of Parkinson's disease: an update". Expert Opinion on Pharmacotherapy. 16 (14): 2231–41. doi:10.1517/14656566.2015.1086748. PMID 26364897. S2CID 6823552.
- 1 2 Poewe W, Mahlknecht P, Krismer F (September 2015). "Therapeutic advances in multiple system atrophy and progressive supranuclear palsy". Movement Disorders. 30 (11): 1528–38. doi:10.1002/mds.26334. PMID 26227071. S2CID 30312372.
- 1 2 3 4 5 6 7 8 9 10 Azilect Prescribing Information Label last revised May, 2014
- 1 2 Richard B. Silverman, Mark W. Holladay. The Organic Chemistry of Drug Design and Drug Action, 3rd Edition. Academic Press, 2014 ISBN 9780123820310
- ↑ Cho HU, Kim S, Sim J, Yang S, An H, Nam MH, et al. (July 2021). "Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis". Experimental & Molecular Medicine. 53 (7): 1148–1158. doi:10.1038/s12276-021-00646-3. PMC 8333267. PMID 34244591. S2CID 235786369.
- ↑ Engberg G, Elebring T, Nissbrandt H (November 1991). "Deprenyl (selegiline), a selective MAO-B inhibitor with active metabolites; effects on locomotor activity, dopaminergic neurotransmission and firing rate of nigral dopamine neurons". The Journal of Pharmacology and Experimental Therapeutics. 259 (2): 841–7. PMID 1658311.
- 1 2 Lemke TL, Williams DA, eds. (2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 434. ISBN 978-1609133450.
- ↑ Bar Am O, Amit T, Youdim MB (January 2004). "Contrasting neuroprotective and neurotoxic actions of respective metabolites of anti-Parkinson drugs rasagiline and selegiline". Neuroscience Letters. 355 (3): 169–72. doi:10.1016/j.neulet.2003.10.067. PMID 14732458. S2CID 20471004.
- ↑ Yasar S, Goldberg JP, Goldberg SR (January 1996). "Are metabolites of l-deprenyl (Selegiline) useful or harmful? Indications from preclinical research". Deprenyl — Past and Future. Vol. 48. pp. 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
{{cite book}}:|journal=ignored (help) - ↑ Chen JJ, Swope DM (August 2005). "Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease". Journal of Clinical Pharmacology. 45 (8): 878–94. doi:10.1177/0091270005277935. PMID 16027398. S2CID 24350277. Archived from the original on 2012-07-11. Retrieved 2008-08-18.
- 1 2 Schapira A, Bate G, Kirkpatrick P (August 2005). "Rasagiline". Nature Reviews. Drug Discovery. 4 (8): 625–6. doi:10.1038/nrd1803. PMID 16106586.
- ↑ Binda C, Hubálek F, Li M, Herzig Y, Sterling J, Edmondson DE, Mattevi A (December 2005). "Binding of rasagiline-related inhibitors to human monoamine oxidases: a kinetic and crystallographic analysis". Journal of Medicinal Chemistry. 48 (26): 8148–54. doi:10.1021/jm0506266. PMC 2519603. PMID 16366596.
- ↑ Lecht S, Haroutiunian S, Hoffman A, Lazarovici P (June 2007). "Rasagiline - a novel MAO B inhibitor in Parkinson's disease therapy". Therapeutics and Clinical Risk Management. 3 (3): 467–74. PMC 2386362. PMID 18488080.
- ↑ US 3201470, Huebner CF, issued 17 August 1965, assigned to CIBA
- ↑ Huebner CF, Donoghue EM, Plummer AJ, Furness PA (November 1966). "N-methyl-n-2-propynyl-l-indanamine. A protent monoamine oxidase inhibitor". Journal of Medicinal Chemistry. 9 (6): 830–2. doi:10.1021/jm00324a009. PMID 5972038.
- ↑ Youdim MB, Bakhle YS (January 2006). "Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness". British Journal of Pharmacology. 147 (Suppl 1): S287–96. doi:10.1038/sj.bjp.0706464. PMC 1760741. PMID 16402116.
- 1 2 US 3513244, Gittos MW, James JW, Wiggins LF, "Methods of lowering blood pressure in animals by administering secondary and tertiary amines", issued 19 May 1970, assigned to Aspro Nicholas Ltd. 5453446 was the patent at issue in "Teva v Watson" (PDF). Archived from the original (PDF) on 23 April 2016.
- ↑ Sielg-Itzkovich J (13 November 2010). "Making armor for the brain". The Jerusalem Post.
- 1 2 3 Finberg JP, Lamensdorf I, Commissiong JW, Youdim MB (1996). "Pharmacology and neuroprotective properties of rasagiline". Deprenyl — Past and Future. Vol. 48. pp. 95–101. doi:10.1007/978-3-7091-7494-4_9. ISBN 978-3-211-82891-5. PMID 8988465.
{{cite book}}:|journal=ignored (help) - ↑ Kupsch A (May 2002). "Rasagiline. Teva Pharmaceutical". Current Opinion in Investigational Drugs. 3 (5): 794–7. PMID 12090555.
- ↑ Eisai Press Release. May 15, 2003
- ↑ Sviderski V (19 October 2011). "FDA Advisers Refuse Teva Plea to Expand Azilect Label". Reuters and Haaretz.
- ↑ Katz R, et al. "Peripheral and Central Nervous System Advisory Committee Background Package on Azilect" (PDF). FDA. Retrieved December 7, 2011.
External links
- "Rasagiline". Drug Information Portal. U.S. National Library of Medicine.
- "Rasagiline mesylate". Drug Information Portal. U.S. National Library of Medicine.