 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪˈmɛtɪdiːn/ or /saɪˈmɛtɪdiːn/ |
| Trade names | Tagamet, others |
| Other names | SKF-92334[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682256 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection, intravenous infusion[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–70%[3][4] |
| Protein binding | 13–25%[4][5] |
| Metabolism | Liver[4] |
| Metabolites | • Cimetidine sulfoxide[4] • Hydroxycimetidine[4] • Guanyl urea cimetidine[4] |
| Onset of action | 30 minutes[6] |
| Elimination half-life | 123 minutes (~2 hours)[5] |
| Duration of action | 4–8 hours[2] |
| Excretion | Urine[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.052.012 |
| Chemical and physical data | |
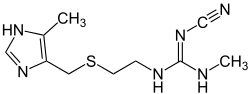
| Formula | C10H16N6S |
| Molar mass | 252.34 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production.[1][7][8] It is mainly used in the treatment of heartburn and peptic ulcers.[1][8][9]
With the development of proton pump inhibitors, such as omeprazole, approved for the same indications, cimetidine is available as an over-the-counter formulation to prevent heartburn or acid indigestion, along with the other H2-receptor antagonists.[10]
Cimetidine was developed in 1971 and came into commercial use in 1977.[11][12] Cimetidine was approved in the United Kingdom in 1976, and was approved in the United States by the Food and Drug Administration in 1979.[13]
Medical uses
Cimetidine is used to inhibit stomach acid production and is used in the treatment of heartburn and peptic ulcers.
Side effects
Reported side effects of cimetidine include diarrhea, rashes, dizziness, fatigue, constipation, and muscle pain, all of which are usually mild and transient.[14] It has been reported that mental confusion may occur in the elderly.[14] Because of its hormonal effects, cimetidine rarely may cause sexual dysfunction including loss of libido and erectile dysfunction and gynecomastia (0.1–0.2%) in males during long-term treatment.[14][15][16] Rarely, interstitial nephritis, urticaria, and angioedema have been reported with cimetidine treatment.[14] Cimetidine is also commonly associated with transient raised aminotransferase activity; hepatotoxicity is rare.[17]
As of May 2021, ranitidine has been withdrawn from the market.[18]
Overdose
Cimetidine appears to be very safe in overdose, producing no symptoms even with massive overdoses (e.g., 20 g).[19]
Interactions
Due to its non-selective inhibition of cytochrome P450 enzymes, cimetidine has numerous drug interactions. Examples of specific interactions include the following:
- Cimetidine affects the metabolism of methadone, sometimes resulting in higher blood levels and a higher incidence of side effects, and may interact with the antimalarial medication hydroxychloroquine.[20]
- Cimetidine can also interact with a number of psychoactive medications, including tricyclic antidepressants and selective serotonin reuptake inhibitors, causing increased blood levels of these drugs and the potential of subsequent toxicity.
- Following administration of cimetidine, the elimination half-life and area-under-curve of zolmitriptan and its active metabolites were roughly doubled.[21]
- Cimetidine is a potent inhibitor of tubular creatinine secretion. Creatinine is a metabolic byproduct of creatine breakdown. Accumulation of creatinine is associated with uremia, but the symptoms of creatinine accumulation are unknown, as they are hard to separate from other nitrogenous waste buildups.[22]
- Like several other medications (e.g. erythromycin), cimetidine interferes with the body's metabolization of sildenafil, causing its strength and duration to increase (therefore also its side effects to be more likely and prominent).
- Clinically significant drug interactions with the CYP1A2 substrate theophylline, the CYP2C9 substrate tolbutamide, the CYP2D6 substrate desipramine, and the CYP3A4 substrate triazolam have all been demonstrated with cimetidine, and interactions with other substrates of these enzymes are likely as well.[23]
- Cimetidine has been shown clinically to reduce the clearance of mirtazapine, imipramine, timolol, nebivolol, sparteine, loratadine, nortriptyline, gabapentin, and desipramine in humans.[24]
- Cimetidine inhibits the renal excretion of metformin and procainamide, resulting in increased circulating levels of these drugs.[14]
- Interactions of potential clinical importance with cimetidine include warfarin, theophylline, phenytoin, carbamazepine, pethidine and other opioid analgesics, tricyclic antidepressants, lidocaine, terfenadine, amiodarone, flecainide, quinidine, fluorouracil, and benzodiazepines.[14][25]
- Cimetidine may decrease the effects of CYP2D6 substrates that are prodrugs, such as codeine, tramadol, and tamoxifen.[26]
- Cimetidine reduces the absorption of ketoconazole and itraconazole (which require a low pH).[14]
- Cimetidine has a theoretical but unproven benefit in paracetamol toxicity.[17] This is because N-acetyl-p-benzoquinone imine (NAPQI), a metabolite of paracetamol (acetaminophen) that is responsible for its hepatotoxicity, is formed from it by the cytochrome P450 system (specifically, CYP1A2, CYP2E1, and CYP3A4).[27]
- Used in cancer metastasis research as a blocker of E-selectin. [28]
- Numerous other drug interactions.
Pharmacology
Pharmacodynamics
Histamine H2 receptor antagonism
The mechanism of action of cimetidine as an antacid is as a histamine H2 receptor antagonist.[29] It has been found to bind to the H2 receptor with a Kd of 42 nM.[30]
Cytochrome P450 inhibition
Cimetidine is a potent inhibitor of certain cytochrome P450 (CYP) enzymes,[19][31] including CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.[19][31][32] The drug appears to primarily inhibit CYP1A2, CYP2D6, and CYP3A4,[33] of which it is described as a moderate inhibitor.[6] This is notable since these three CYP isoenzymes are involved in CYP-mediated drug biotransformations;[34] however, CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 are also involved in the oxidative metabolism of many commonly used drugs.[35] As a result, cimetidine has the potential for a large number of pharmacokinetic interactions.[19][31][32]
Cimetidine is reported to be a competitive and reversible inhibitor of several CYP enzymes,[17][25][31][36] although mechanism-based (suicide) irreversible inhibition has also been identified for cimetidine's inhibition of CYP2D6.[24] It reversibly inhibits CYP enzymes by binding directly with the complexed heme-iron of the active site via one of its imidazole ring nitrogen atoms, thereby blocking the oxidation of other drugs.[31][36][37]
Antiandrogenic and estrogenic effects
Cimetidine has been found to possess weak antiandrogenic activity at high doses.[29][38][39][40] It directly and competitively antagonizes the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[41][42] However, the affinity of cimetidine for the AR is very weak; in one study, it showed only 0.00084% of the affinity of the anabolic steroid metribolone (100%) for the human AR (Ki = 140 μM and 1.18 nM, respectively).[43] In any case, at sufficiently high doses, cimetidine has demonstrated weak but significant antiandrogenic effects in animals, including antiandrogenic effects in the rat ventral prostate and mouse kidney, reductions in the weights of the male accessory glands like the prostate gland and seminal vesicles in rats, and elevated gonadotropin levels in male rats (due to reduced negative feedback on the HPG axis by androgens).[44][45] In addition to AR antagonism, cimetidine has been found to inhibit the 2-hydroxylation of estradiol (via inhibition of CYP450 enzymes, which are involved in the metabolic inactivation of estradiol), resulting in increased estrogen levels.[46][47][48][49][50] The medication has also been reported to reduce testosterone biosynthesis and increase prolactin levels in individual case reports, effects which might be secondary to increased estrogen levels.[51]
At typical therapeutic levels, cimetidine has either no effect on or causes small increases in circulating testosterone concentrations in men.[44] Any increases in testosterone levels with cimetidine have been attributed to the loss of negative feedback on the HPG axis that results due to AR antagonism.[44][45] At typical clinical dosages, such as those used to treat peptic ulcer disease, the incidence of gynecomastia (breast development) with cimetidine is very low at less than 1%.[52][44] In one survey of over 9,000 patients taking cimetidine, gynecomastia was the most frequent endocrine-related complaint but was reported in only 0.2% of patients.[44] At high doses however, such as those used to treat Zollinger–Ellison syndrome, there may be a higher incidence of gynecomastia with cimetidine.[52] In one small study, a 20% incidence of gynecomastia was observed in 25 male patients with duodenal ulcers who were treated with 1,600 mg/day cimetidine.[51] The symptoms appeared after 4 months of treatment and regressed within a month following discontinuation of cimetidine.[51] In another small study, cimetidine was reported to have induced breast changes and erectile dysfunction in 60% of 22 men treated with it.[51] These adverse effects completely resolved in all cases when the men were switched from cimetidine to ranitidine.[51] A study of the United Kingdom General Practice Research Database, which contains over 80,000 men, found that the relative risk of gynecomastia in cimetidine users was 7.2 relative to non-users.[51] People taking a dosage of cimetidine of greater than or equal to 1,000 mg showed more than 40 times the risk of gynecomastia than non-users.[51] The risk was highest during the period of time of 7 to 12 months after starting cimetidine.[51] The gynecomastia associated with cimetidine is thought to be due to blockade of ARs in the breasts, which results in estrogen action unopposed by androgens in this tissue, although increased levels of estrogens due to inhibition of estrogen metabolism is another possible mechanism.[51] Cimetidine has also been associated with oligospermia (decreased sperm count) and sexual dysfunction (e.g., decreased libido, erectile dysfunction) in men in some research, which are hormonally related similarly.[45][44][51]
In accordance with the very weak nature of its AR antagonistic activity, cimetidine has shown minimal effectiveness in the treatment of androgen-dependent conditions such as acne, hirsutism (excessive hair growth), and hyperandrogenism (high androgen levels) in women.[53][54][52][55] As such, its use for such indications is not recommended.[54][55]
Pharmacokinetics
Cimetidine is rapidly absorbed regardless of route of administration.[5] The oral bioavailability of cimetidine is 60 to 70%.[3][4] The onset of action of cimetidine when taken orally is 30 minutes,[6] and peak levels occur within 1 to 3 hours.[3] Cimetidine is widely distributed throughout all tissues.[5] It is able to cross the blood–brain barrier and can produce effects in the central nervous system (e.g., headaches, dizziness, somnolence).[2] The volume of distribution of cimetidine is 0.8 L/kg in adults and 1.2 to 2.1 L/kg in children.[4] Its plasma protein binding is 13 to 25% and is said to be without pharmacological significance.[4][5] Cimetidine undergoes relatively little metabolism, with 56 to 85% excreted unchanged.[5] It is metabolized in the liver into cimetidine sulfoxide, hydroxycimetidine, and guanyl urea cimetidine.[4] The major metabolite of cimetidine is the sulfoxide, which accounts for about 30% of excreted material.[5] Cimetidine is rapidly eliminated, with an elimination half-life of 123 minutes, or about 2 hours.[5] It has been said to have a duration of action of 4 to 8 hours.[2] The medication is mainly eliminated in urine.[5]
History
Cimetidine, approved by the FDA for inhibition of gastric acid secretion, has been advocated for a number of dermatological diseases.[56] Cimetidine was the prototypical histamine H2 receptor antagonist from which the later members of the class were developed. Cimetidine was the culmination of a project at Smith, Kline and French (SK&F) Laboratories in Welwyn Garden City (now part of GlaxoSmithKline) by James W. Black, C. Robin Ganellin, and others to develop a histamine receptor antagonist to suppress stomach acid secretion.[57] This was one of the first drugs discovered using a rational drug design approach. Sir James W. Black shared the 1988 Nobel Prize in Physiology or Medicine for the discovery of propranolol and also is credited for the discovery of cimetidine.
At the time (1964), histamine was known to stimulate the secretion of stomach acid, but also that traditional antihistamines had no effect on acid production. In the process, the SK&F scientists also proved the existence of histamine H2 receptors.
The SK&F team used a rational drug-design structure starting from the structure of histamine — the only design lead, since nothing was known of the then hypothetical H2 receptor. Hundreds of modified compounds were synthesized in an effort to develop a model of the receptor. The first breakthrough was Nα-guanylhistamine, a partial H2 receptor antagonist. From this lead, the receptor model was further refined and eventually led to the development of burimamide, the first H2 receptor antagonist. Burimamide, a specific competitive antagonist at the H2 receptor, 100 times more potent than Nα-guanylhistamine, proved the existence of the H2 receptor.
Burimamide was still insufficiently potent for oral administration, and further modification of the structure, based on modifying the pKa of the compound, led to the development of metiamide. Metiamide was an effective agent; it was associated, however, with unacceptable nephrotoxicity and agranulocytosis.[57] The toxicity was proposed to arise from the thiourea group, and similar guanidine analogues were investigated until the ultimate discovery of cimetidine. The compound was synthesized in 1972 and evaluated for toxicology by 1973. It passed all trials.
Cimetidine was first marketed in the United Kingdom in 1976, and in the U.S. in August 1977; therefore, it took 12 years from initiation of the H2 receptor antagonist program to commercialization. By 1979, Tagamet was being sold in more than 100 countries and became the top-selling prescription product in the U.S., Canada, and several other countries. In November 1997, the American Chemical Society and the Royal Society of Chemistry in the U.K. jointly recognized the work as a milestone in drug discovery by designating it an International Historic Chemical Landmark during a ceremony at SmithKline Beecham's New Frontiers Science Park research facilities in Harlow, England.[58]
The commercial name "Tagamet" was decided upon by fusing the two words "antagonist" and "cimetidine".[57] Subsequent to the introduction onto the U.S. drug market, two other H2 receptor antagonists were approved, ranitidine (Zantac, Glaxo Labs) and famotidine (Pepcid, Yamanouchi, Ltd.) Cimetidine became the first drug ever to reach more than $1 billion a year in sales, thus making it the first blockbuster drug.
Tagamet has been largely replaced by proton pump inhibitors for treating peptic ulcers, but is available as an over-the-counter medicine for heartburn in many countries.[58]
Research
Some evidence suggests cimetidine could be effective in the treatment of common warts, but more rigorous double-blind clinical trials found it to be no more effective than a placebo.[59][60][61]
Tentative evidence supports a beneficial role as add-on therapy in colorectal cancer.[62]
Cimetidine inhibits ALA synthase activity and hence may have some therapeutic value in preventing and treating acute porphyria attacks.[63][64]
There is some evidence supporting the use of Cimetidine in the treatment of PFAPA.[65]
References
- 1 2 3 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Bibliographies. Springer. pp. 275–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 Gupta A, Singh-Radcliff N (12 March 2013). Pharmacology in Anesthesia Practice. Oxford University Press. pp. 177–. ISBN 978-0-19-934399-7.
- 1 2 3 Dowd FJ, Johnson B, Mariotti A (3 September 2016). Pharmacology and Therapeutics for Dentistry - E-Book. Elsevier Health Sciences. pp. 406–. ISBN 978-0-323-44595-5.
- 1 2 3 4 5 6 7 8 9 10 Leikin JB, Paloucek FP (1995). Poisoning & Toxicology Handbook, 1995-1996. Lexi-Comp, Incorporated. p. 184. ISBN 978-0-916589-08-0.
- 1 2 3 4 5 6 7 8 9 10 Profiles of Drug Substances, Excipients and Related Methodology. Academic Press. 24 October 1984. pp. 176–. ISBN 978-0-08-086108-1.
- 1 2 3 Vallerand AH, Sanoski CA, Deglin JH (25 May 2016). Davis's Drug Guide for Nurses. F.A. Davis. pp. 636–. ISBN 978-0-8036-5779-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 234–. ISBN 978-3-88763-075-1.
- 1 2 Morton I, Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 77–. ISBN 978-0-7514-0499-9.
- ↑ Jacqueline B, Rosenthal L (2 December 2014). "Drugs for Peptic Ulcer Disease". Lehne's Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 952–. ISBN 978-0-323-34026-7.
- ↑ Pino MA, Azer SA (March 2023). "Cimetidine". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. PMID 31334975. Bookshelf ID: NBK544255. Retrieved 6 November 2023 – via U.S. National Library of Medicine.
- ↑ Fischer J, Ganellin CR (24 August 2010). Analogue-based Drug Discovery II. John Wiley & Sons. p. 4. ISBN 978-3-527-63212-1.
- ↑ Alapi EM, Fischer J (2006). "Table of Selected Analogue Classes". In Fischer J, Ganellin CR (eds.). Analogue-based Drug Discovery. John Wiley & Sons. p. 444. ISBN 9783527607495.
- ↑ "Tagamet: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 9 November 2023.
- 1 2 3 4 5 6 7 Ritter J, Lewis L, Mant T, Ferro A (25 April 2008). "Alimenary System and Liver". A Textbook of Clinical Pharmacology and Therapeutics (5th ed.). CRC Press. pp. 250–. ISBN 978-1-4441-1300-6.
- ↑ Sawyer D, Conner CS, Scalley R (February 1981). "Cimetidine: adverse reactions and acute toxicity". American Journal of Hospital Pharmacy. 38 (2): 188–197. PMID 7011006.
- ↑ Sabesin SM (1993). "Safety issues relating to long-term treatment with histamine H2-receptor antagonists". Alimentary Pharmacology & Therapeutics. 7 (Suppl 2): 35–40. doi:10.1111/j.1365-2036.1993.tb00597.x. PMID 8103374. S2CID 42564864.
- 1 2 3 Murray KF (26 January 2009). "Drug-Induced Liver Disease". In Kelly D (ed.). Diseases of the Liver and Biliary System in Children. John Wiley & Sons. pp. 224–. ISBN 978-1-4443-0054-3.
- ↑ Wagner JA, Dinh JC, Lightdale JR, Gold BD, Colombo JM (July 2021). "Is this the end for ranitidine? NDMA presence continues to confound". Clinical and Translational Science. 14 (4): 1197–1200. doi:10.1111/cts.12995. PMC 8301580. PMID 33934515.
- 1 2 3 4 Whyte IM (2004). "Histaminde H2 Antagonists". In Dart RC (ed.). Medical Toxicology. Lippincott Williams & Wilkins. pp. 402–. ISBN 978-0-7817-2845-4.
- ↑ Furst DE (June 1996). "Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases". Lupus. 5 (Suppl 1): S11–S15. doi:10.1177/096120339600500104. PMID 8803904. S2CID 44999237.
- ↑ See complete drug interactions for Zomig (zolmitriptan succinate used for migraine relief) in package insert: "Highlights of Zomig Prescribing Information" (PDF). AstraZeneca. Archived from the original (PDF) on 18 February 2015. Retrieved 28 January 2010.
- ↑ Urakami Y, Kimura N, Okuda M, Masuda S, Katsura T, Inui K (June 2005). "Transcellular transport of creatinine in renal tubular epithelial cell line LLC-PK1". Drug Metabolism and Pharmacokinetics. 20 (3): 200–205. doi:10.2133/dmpk.20.200. PMID 15988122. S2CID 13857940.
- ↑ Ogilvie BW, Usuki E, Yerino P, Parkinson A (8 February 2008). "In vitro approaches for studying the inhibition of drug-metabolizing enzymes and identifying the drug-metabolizing enzymes responsible for the metabolism of drugs (reaction phenotyping) with emphasis on cytochrome P450". In Rodrigues DA (ed.). Drug-Drug Interactions (Second ed.). CRC Press. pp. 277, 294. ISBN 978-0-8493-7594-1.
- 1 2 Zhou S (6 April 2016). "Inhibitors of Human CYP2D6". Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. pp. 299–. ISBN 978-1-4665-9788-4.
- 1 2 Rosenfeld GC, Loose DS (2007). Pharmacology. Lippincott Williams & Wilkins. pp. 202–. ISBN 978-0-7817-8074-2.
- ↑ Fuller MA, Sajatovic M (2005). Drug Information Handbook for Psychiatry. Lexi-Comp. p. 285. ISBN 9781591951148.
- ↑ Leeder JS, Okey AB (6 December 2012). "Cytochrome P450 and Liver injury". In Cameron R, Feuer G, de la Iglesias F (eds.). Drug-Induced Hepatotoxicity. Springer Science & Business Media. pp. 140–. ISBN 978-3-642-61013-4.
- ↑ Coppo R, Orso F, Virga F, Dalmasso A, Baruffaldi D, Nie L, et al. (July 2021). "ESDN inhibits melanoma progression by blocking E-selectin expression in endothelial cells via STAT3". Cancer Letters. 510: 13–23. doi:10.1016/j.canlet.2021.04.005. hdl:2318/1791282. PMC 8581997. PMID 33862151.
{{cite journal}}: CS1 maint: overridden setting (link) - 1 2 Richards DA (1983). "Comparative pharmacodynamics and pharmacokinetics of cimetidine and ranitidine". Journal of Clinical Gastroenterology. 5 (Suppl 1): 81–90. doi:10.1097/00004836-198312001-00008. PMID 6317740. S2CID 24909853.
- ↑ Taylor JE (22 October 2013). Osborne NN (ed.). "Neurochemical and neuropharmacological aspects of histamine receptors". Neurochemistry International. Elsevier Science. 4 (2–3): 89–96. doi:10.1016/0197-0186(82)90001-8. ISBN 978-1-4832-8635-8. PMID 20487855. S2CID 40069290.
- 1 2 3 4 5 Williams DA (2008). "Drug Metabolism". In Lemke TL, Williams DA (eds.). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 273–. ISBN 978-0-7817-6879-5.
- 1 2 Karalliedde LD, Clarke SF, Collignon U, Karalliedde J (29 January 2010). "Drugs Acting on the Gastrointestinal Track". Adverse Drug Interactions: A Handbook for Prescribers. CRC Press. pp. 633–. ISBN 978-0-340-92769-4.
- ↑ Priskorn M, Larsen F, Segonzac A, Moulin M (1997). "Pharmacokinetic interaction study of citalopram and cimetidine in healthy subjects". European Journal of Clinical Pharmacology. 52 (3): 241–242. doi:10.1007/s002280050282. PMID 9218934. S2CID 22540140.
- ↑ Martínez C, Albet C, Agúndez JA, Herrero E, Carrillo JA, Márquez M, et al. (April 1999). "Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists". Clinical Pharmacology and Therapeutics. 65 (4): 369–376. doi:10.1016/S0009-9236(99)70129-3. PMID 10223772. S2CID 25151710.
{{cite journal}}: CS1 maint: overridden setting (link) - ↑ Delafuente JC (November 2003). "Understanding and preventing drug interactions in elderly patients". Critical Reviews in Oncology/Hematology. 48 (2): 133–143. doi:10.1016/j.critrevonc.2003.04.004. PMID 14607376.
- 1 2 Cairns D (2012). "Drug Metabolism: Drug Conjugating Reactions (Phase 2)". Essentials of Pharmaceutical Chemistry. Pharmaceutical Press. pp. 110–. ISBN 978-0-85369-979-8.
Drugs interacting in this way with CYP include the histamine H2-receptor antagonist cimetidine, [...] Reversible inhibitors, such as cimetidine, which interact with the complexed iron at the active site of the enzyme to inhibit oxidation of other drugs. The inhibition occurs before any oxidation of the inhibitor occurs and is reversible once the inhibitor is removed.
- ↑ Liska DJ (June 1998). "The detoxification enzyme systems". Alternative Medicine Review. 3 (3): 187–198. PMID 9630736.
Cimetidine is an example of a compound that can bind directly to the heme iron of the cytochrome P450 reactive site to inhibit all cytochrome-dependent Phase I enzyme activities.13
- ↑ Matsumoto AM (2001). "Clinical Use and Abuse of Androgens and Antiandrogens". In Becker KL (ed.). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1196–. ISBN 978-0-7817-1750-2.
- ↑ Jensen RT, Collen MJ, McArthur KE, Howard JM, Maton PN, Cherner JA, et al. (November 1984). "Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric hypersecretory states". The American Journal of Medicine. 77 (5B): 90–105. PMID 6150641.
- ↑ Biagi P, Milani G (March 1985). "[Dysfunction of the hypothalamo-hypophyseal-gonadal axis induced by histamine H2 antagonists. Review of the literature and personal observations]". Minerva Medica (in Italian). 76 (12): 579–586. PMID 3921876.
- ↑ Winters SJ, Banks JL, Loriaux DL (March 1979). "Cimetidine is an antiandrogen in the rat". Gastroenterology. 76 (3): 504–508. doi:10.1016/S0016-5085(79)80217-6. PMID 428705.
- ↑ Sivelle PC, Underwood AH, Jelly JA (March 1982). "The effects of histamine H2 receptor antagonists on androgen action in vivo and dihydrotestosterone binding to the rat prostate androgen receptor in vitro". Biochemical Pharmacology. 31 (5): 677–684. doi:10.1016/0006-2952(82)90449-X. PMID 6123322.
- ↑ Eil C, Edelson SK (July 1984). "The use of human skin fibroblasts to obtain potency estimates of drug binding to androgen receptors". The Journal of Clinical Endocrinology and Metabolism. 59 (1): 51–55. doi:10.1210/jcem-59-1-51. PMID 6725525.
- 1 2 3 4 5 6 Ward OB (11 November 2013). "Fetal drug exposure and sexual differentiation of males.". In Gerall AA, Moltz H, Ward EL (eds.). Sexual Differentiation. Springer Science & Business Media. pp. 207–. ISBN 978-1-4899-2453-7.
In high concentrations cimetidine acts as a weak antiandrogen by competitively binding to cytosol androgen receptors, as has been demonstrated in rat ventral prostate (Foldesy, Vanderhoof, & Hahn, 1985; Sivelle, Underwood, & Jelly, 1982) and mouse kidney tissue (Funder & Mercer, 1979). In vivo, cimetidine, in high dose levels, causes reductions in prostate and seminal vesicle weights in male rats (Foldesy et al., 1985; Leslie & Walker, 1977; Sivelle et al., 1982). After 6 weeks of daily cimetidine administration to male rats, reduced weights of accessory sexual organs were accompanied by elevated gonadotropin levels (Baba, Paul, Pollow, Janetschek, & Jacobi, 1981). At therapeutic levels in men, cimetidine either has no effect on plasma T levels (Spona et al., 1987; Stubbs et al., 1983) or causes small increases in T (Peden, Boyd, Browning, Saunders, & Wormsley, 1981; Van Thiel, Gavaler, Smith, & Paul, 1979; Wang, Lai, Lam, & Yeung, 1982). The increases in T have been attributed to cimetidine's antagonism of the normal negative feedback that androgens exert on gonadotropin secretion (Peden, Cargill, Browning, Saunders, & Wormsley, 1979). Gynecomastia and even loss of libido that progressed to impotence have occasionally been reported in men taking cimetidine (Peden et al., 1979; Spence & Celestin, 1979), but the occurrence of these disorders is very rare (Gifford, Aeugle, Myerson, & Tannenbaum, 1980). In one survey, gynecomastia, the most frequent endocrine-related complaint, was reported in only 0.2% of over 9,000 patients taking cimetidine (Gifford et al., 1980).
- 1 2 3 Barazani Y, Sabanegh Jr ES (26 July 2014). "Risks from Medical and Therapeutic Treatments". In du Plessis SS, Agarwal A, Sabanegh Jr ES (eds.). Male Infertility: A Complete Guide to Lifestyle and Environmental Factors. Springer. pp. 233–. ISBN 978-1-4939-1040-3.
Like other antiandrogens, [cimetidine] leads to elevated gonadotropin levels by antagonizing the negative feedback control of gonadotropin secretion by testosterone [1, 34]. Cimetidine has been reported to have antiandrogenic effects ranging from gynecomastia to oligospermia [4]. In one clinical study, men administered cimetidine exhibited a significant reduction in sperm concentration compared to placebo-treated controls [35]. In another study of men receiving cimetidine for chronic duodenal ulcers, testosterone and FSH were elevated during treatment with cimetidine compared to both pre- and posttreatment levels. Moreover, these hormonal effects were associated with a reduction in mean sperm count compared to the period after drug withdrawal [34].
- ↑ Galbraith RA, Michnovicz JJ (August 1989). "The effects of cimetidine on the oxidative metabolism of estradiol". The New England Journal of Medicine. 321 (5): 269–274. doi:10.1056/NEJM198908033210501. PMID 2747769.
- ↑ Michnovicz JJ, Galbraith RA (February 1991). "Cimetidine inhibits catechol estrogen metabolism in women". Metabolism. 40 (2): 170–174. doi:10.1016/0026-0495(91)90169-W. PMID 1988774.
- ↑ Pescovitz OH, Walvoord EC (6 June 2007). When Puberty is Precocious: Scientific and Clinical Aspects. Springer Science & Business Media. pp. 203–. ISBN 978-1-59745-499-5.
- ↑ Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B (March 2014). "Gynecomastia: Clinical evaluation and management". Indian Journal of Endocrinology and Metabolism. 18 (2): 150–158. doi:10.4103/2230-8210.129104. PMC 3987263. PMID 24741509.
- ↑ Rendic S, Di Carlo FJ (2010). "Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors". Drug Metabolism Reviews. 29 (1–2): 413–580. doi:10.3109/03602539709037591. PMID 9187528.
- 1 2 3 4 5 6 7 8 9 10 Deepinder F, Braunstein GD (September 2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–795. doi:10.1517/14740338.2012.712109. PMID 22862307. S2CID 22938364.
Cimetidine. Spence and Celestin reported a 20% incidence of gynecomastia in a prospective study of 25 male duodenal ulcer patients treated with cimetidine 1.6 g/day [13]. Symptoms developed after 4 months of treatment and regressed within a month of stopping therapy. In another prospective cohort study involving 22 patients, cimetidine caused breast changes and erectile dysfunction in 60% of men which resolved completely in all cases when switched to ranitidine [14]. In the UK general practice database of over 80,000 men, the relative risk (RR) of gynecomastia among cimetidine users was 7.2 (95% confidence interval (CI 4.5 -- 11.3)) as compared with the non-users. Users with a daily dose ‡ 1000 mg had more than 40 times the risk of developing gynecomastia than the non-users. The period of highest risk was 7 -- 12 months after starting cimetidine treatment [15]. Cimetidine blocks the androgen receptors in the breast leading to decreased androgen action causing the growth of breast tissue because of 'unopposed' estrogen action [16]. Another possible mechanism includes decreased 2-hydroxylation of estrogen leading to elevated serum estrogen levels [17]. There also are reports of cimetidine blocking testosterone biosynthesis and causing elevated prolactin levels in individual cases [18].
- 1 2 3 Dunaway G (1 April 2009). "Androgens and Antiandrogens". In Watts S, Faingold C, Dunaway G, Crespo L (eds.). Brody's Human Pharmacology - E-Book. Elsevier Health Sciences. pp. 472–. ISBN 978-0-323-07575-6.
The histamine receptor antagonist cimetidine, used to decrease gastric acid secretion in treatment of peptic ulcer disease and esophagitis (see Chapter 14), also acts as an antiandrogen. Thus it has been reported to produce gynecomastia when given in large doses, such as those used in the treatment of patients with Zollinger-Ellison syndrome. Gynecomastia occurs in less than 1% of patients treated with the doses used in peptic ulcer disease. Cimetidine interacts with ARs approximately 0.01% as effectively as testosterone and has been used with limited effectiveness to treat hirsutism in women.
- ↑ Copperman AB, Mukherjee T, Kase NG (4 September 2003). "Polycystic Ovarian Syndrome". In Altchek A, Deligdisch L, Kase N (eds.). Diagnosis and Management of Ovarian Disorders. Academic Press. pp. 351–. ISBN 978-0-08-049451-7.
Cimetidine is a weak androgen receptor antagonist. A controlled clinical study has not found cimetidine to be effective in the treatment of hyperandrogenism.[123, 124] 5.
- 1 2 Pregler JP, DeCherney AH (2002). "Approach to the Patient with Hirsutism". Women's Health: Principles and Clinical Practice. PMPH-USA. pp. 595–. ISBN 978-1-55009-170-0.
Cimetidine is a histamine type 2 blocker, which also binds to the androgen receptor to inhibit its function." However, this antiandrogen activity of cimetidine is weak, and the clinical benefit of its use in women with hirsutism is minimal. Thus, this drug is not recommended for the treatment of hyperandrogenism.
- 1 2 Katsambas AD, Dessinioti C (2010). "Hormonal therapy for acne: why not as first line therapy? facts and controversies". Clinics in Dermatology. 28 (1): 17–23. doi:10.1016/j.clindermatol.2009.03.006. PMID 20082945.
- ↑ Scheinfeld N (March 2003). "Cimetidine: a review of the recent developments and reports in cutaneous medicine". Dermatology Online Journal. 9 (2): 4. doi:10.5070/D33S15Q645. PMID 12639457.
- 1 2 3 "Tagamet: Discovery of Histamine H2-receptor Antagonists". National Historic Chemical Landmarks. American Chemical Society. Archived from the original on 9 December 2012. Retrieved 25 June 2012.
- 1 2 Fremantle M. "Tagamet". Chemical and Engineering news. Retrieved 1 July 2013.
- ↑ Fit KE, Williams PC (July 2007). "Use of histamine2-antagonists for the treatment of verruca vulgaris". The Annals of Pharmacotherapy. 41 (7): 1222–1226. doi:10.1345/aph.1H616. PMID 17535844. S2CID 19769702.
- ↑ Glass AT, Solomon BA (June 1996). "Cimetidine therapy for recalcitrant warts in adults". Archives of Dermatology. 132 (6): 680–682. doi:10.1001/archderm.1996.03890300108014. PMID 8651718.
- ↑ Karabulut AA, Sahin S, Ekşioglu M (April 1997). "Is cimetidine effective for nongenital warts: a double-blind, placebo-controlled study". Archives of Dermatology. 133 (4): 533–534. doi:10.1001/archderm.133.4.533. PMID 9126017.
- ↑ Deva S, Jameson M (August 2012). "Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer". The Cochrane Database of Systematic Reviews. 8 (8): CD007814. doi:10.1002/14651858.CD007814.pub2. PMID 22895966.
- ↑ Whatley SD, Badminton MN (2013). Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al. (eds.). Acute Intermittent Porphyria. University of Washington, Seattle. PMID 20301372.
- ↑ Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J, et al. (APM:SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine) (2015). Acute Pain Management: Scientific Evidence (4th ed.). Melbourne, Australia: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. p. 316. ISBN 978-0-9873236-6-8. Archived from the original (PDF) on 31 July 2019. Retrieved 7 September 2017.
- ↑ Vanoni F, Theodoropoulou K, Hofer M (June 2016). "PFAPA syndrome: a review on treatment and outcome". Pediatric Rheumatology Online Journal. 14 (1): 38. doi:10.1186/s12969-016-0101-9. PMC 4924332. PMID 27349388.
External links
 Media related to Cimetidine at Wikimedia Commons
Media related to Cimetidine at Wikimedia Commons