 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
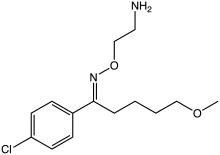
| Formula | C14H21ClN2O2 |
| Molar mass | 284.78 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Clovoxamine (INN) (developmental code name DU-23811) is a drug that was discovered in the 1970s and was subsequently investigated as an antidepressant and anxiolytic agent but was never marketed.[1][2][3] It acts as a serotonin-norepinephrine reuptake inhibitor (SNRI), with little affinity for the muscarinic acetylcholine, histamine, adrenergic, and serotonin receptors.[4][5] The compound is structurally related to fluvoxamine.
References
- ↑ Freeman HL, Wakelin JS, Calanca A, Hole G (1982). "Initial clinical evaluation of a new nontricyclic antidepressant: clovoxamine". Advances in Biochemical Psychopharmacology. 32: 69–75. PMID 7046368.
- ↑ Jesinger DK, Gostick N (October 1989). "Anxiety neurosis in general practice. A double-blind comparative study of diazepam and clovoxamine, a novel inhibitor of noradrenaline and serotonin reuptake". International Clinical Psychopharmacology. 4 (4): 301–11. doi:10.1097/00004850-198910000-00005. PMID 2691573.
- ↑ Hurst HE, Jones DR, Wright JH, Jarboe CH (August 1983). "Clovoxamine kinetics in an early clinical trial". Clinical Pharmacology and Therapeutics. 34 (2): 266–71. doi:10.1038/clpt.1983.164. PMID 6872422. S2CID 42205772.
- ↑ Saletu B, Grünberger J, Rajna P, Karobath M (1980). "Clovoxamine and fluvoxamine-2 biogenic amine re-uptake inhibiting antidepressants: quantitative EEG, psychometric and pharmacokinetic studies in man". Journal of Neural Transmission. 49 (1–2): 63–86. doi:10.1007/BF01249190. PMID 6777458. S2CID 6483654.
- ↑ Bradford LD, Tulp MT, Schipper J (June 1987). "Biochemical effects in rats after acute and long-term treatment with clovoxamine". Archives Internationales de Pharmacodynamie et de Therapie. 287 (2): 188–202. PMID 2820327.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.