 | |
-Phenylephrine_molecule_ball.png.webp) | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌfɛnəlˈɛfriːn, fiː-, -ɪn/ |
| Trade names | Neo-synephrine, others[1] |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, in the nose, on the eye, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 38% through GI tract |
| Protein binding | 95% |
| Metabolism | Liver (oxidative deamination) |
| Onset of action | Very rapid (IV); within 20 min (by mouth)[2] |
| Elimination half-life | 2.1–3.4 h |
| Duration of action | Up to 20 min (IV); 4 hrs (by mouth)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.386 |
| Chemical and physical data | |
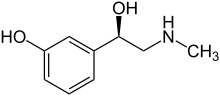
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phenylephrine is a medication used as a decongestant for uncomplicated nasal congestion,[3] used to dilate the pupil, used to increase blood pressure (given intravenously in cases of low blood pressure), and used to relieve hemorrhoids.[2][4] It can be taken by mouth, as a nasal spray, given by injection into a vein or muscle, or applied to the skin.[2]
Common side effects when taken by mouth or injected include nausea, vomiting, headache, and anxiety.[2] Use on hemorrhoids is generally well tolerated.[2] Severe side effects may include a slow heart rate, intestinal ischemia, chest pain, kidney failure, and tissue death at the site of injection.[2][4] It is unclear whether its use during pregnancy and breastfeeding is safe.[2] Phenylephrine is a selective α1-adrenergic receptor agonist with minimal to no β-adrenergic receptor agonist activity.[3] It causes constriction of both arteries and veins.[2]
Phenylephrine was patented in 1933[5] and came into medical use in 1938.[6] It is available as a generic medication.[4][7][8] Unlike pseudoephedrine, abuse of phenylephrine is very uncommon.[9] Its effectiveness as a nasal decongestant has been questioned.[2][10][11] In 2023, a Food and Drug Administration panel concluded that the drug was ineffective as a nasal decongestant when taken orally.[12]
Medical uses
Decongestant
Phenylephrine is used as an alternative to pseudoephedrine as a decongestant, whose availability has been restricted due to a potential for use in the illicit synthesis of methamphetamine.[13] Its efficacy as an oral decongestant has been questioned, with several independent studies finding that it provided no more relief to sinus congestion than a placebo.[14][15][16]
A 2007 meta-analysis concluded that the evidence for its effectiveness is insufficient,[17] though another meta-analysis published shortly thereafter by researchers from GlaxoSmithKline found the standard 10-mg dose to be more effective than a placebo; however, the fact that GSK markets many products containing phenylephrine has raised some speculation regarding selective publishing and other controversial techniques.[18] A 2007 study by Wyeth Consumer Healthcare notes that 7 studies available in 1976 support the efficacy of phenylephrine at a 10 mg dosage.[19] The Food and Drug Administration withdrew the indication "for the temporary relief of nasal congestion associated with sinusitis" in 2007.[2]
Two studies published in 2009, examined the effects of phenylephrine on symptoms of allergic rhinitis by exposing people to pollen in a controlled, indoor environment. Neither study was able to distinguish between the effects of phenylephrine or a placebo. Pseudoephedrine and loratadine–montelukast therapy were found to be significantly more effective than both phenylephrine and placebo.[14][15]
Pseudoephedrine was previously much more commonly available in the United States, however, provisions of the Combat Methamphetamine Epidemic Act of 2005 placed restrictions on the sale of pseudoephedrine products to prevent the clandestine manufacture of methamphetamine. Since 2004, phenylephrine has been increasingly marketed as a substitute for pseudoephedrine; some manufacturers have changed the active ingredients of products to avoid the restrictions on sales.[20] Phenylephrine has been off-patent for some time, and many generic brands are available.
In September 2023, an independent advisory committee to the US Food and Drug Administration (FDA) unanimously agreed that there is insufficient evidence showing that "orally administered phenylephrine is effective as a nasal decongestant".[21] The committee also unanimously believes that this does not need further study. The FDA responded to the committee, stating it would take its advice under advisement.[22][23]
Hemorrhoids
Hemorrhoids are caused by swollen veins in the rectal area.[24] Phenylephrine can be used topically to prevent symptoms of hemorrhoids. Phenylephrine causes the constriction of vascular smooth muscle and is often used in the treatment of hemorrhoids to narrow the swollen veins and relieve the attendant pain. However, veins contain less vascular smooth muscle in their walls than arteries. Products for treatment may also include substances that will form a protective barrier over the inflamed area, resulting in less pain when feces are passed.[25]
Phenylephrine hydrochloride at 0.25% is used as a vasoconstrictor in suppository formulations for hemorrhoid treatment.[26]
Pupil dilation
Phenylephrine is used as an eye drop to dilate the pupil to facilitate visualization of the retina. It is often used in combination with tropicamide as a synergist when tropicamide alone is not sufficient. Narrow-angle glaucoma is a contraindication to phenylephrine use. As a mydriatic, it is available in 2.5% and 10% minims. Phenylephrine eye drops are applied to the eye after a topical anesthetic is applied.[27]
Intraocular bleeding
Phenylephrine has been used as an intracameral injection into the anterior chamber of the eye to arrest intraocular bleeding occurring during cataract and glaucoma surgery.[28]
Vasopressor
Phenylephrine is commonly used as a vasopressor to increase the blood pressure in unstable patients with hypotension, especially resulting from septic shock. Such use is common in anesthesia or critical-care practices; it is especially useful in counteracting the hypotensive effect of epidural and spinal anesthesia, as well as the vasodilating effect of bacterial toxins and the inflammatory response in sepsis and systemic inflammatory response syndrome. The elimination half life of phenylephrine is about 2.5 to 3.0 hours.[29]
Because of its vasoconstrictive effect, phenylephrine can cause severe necrosis if it infiltrates the surrounding tissues. Because of this, it should be given through a central line if at all possible. Damage may be prevented or mitigated by infiltrating the tissue with the alpha blocker phentolamine by subcutaneous injection.[30]
Priapism
Phenylephrine is used to treat priapism. It is diluted with normal saline and injected directly into the corpora cavernosa. The mechanism of action is to cause constriction of the blood vessels entering into the penis, thus causing decreased blood flow and relieving the priapism.
Side effects
Phenylephrine may cause side effects such as headache, reflex bradycardia, excitability, restlessness and cardiac arrhythmias.[2] Phenylephrine is not suggested for use in people with hypertension.[31]
Heart
The primary side effect of phenylephrine is high blood pressure. People with high blood pressure are typically advised to avoid products containing it. Because this medication is a sympathomimetic amine without β-adrenergic receptor agonist activity, it does not increase contractility force and output of the cardiac muscle. It may increase blood pressure resulting in a slow heart rate through stimulation of vascular (likely carotid) baroreceptors. A common side effect during IV administration is reflex bradycardia.[32] The low concentration eye drops do not cause blood pressure changes and the changes with the higher dose drops do not last long.[33]
Other
Prostatic hyperplasia can also be worsened by use, and chronic use can lead to rebound hyperemia.[34] People with a history of anxiety or panic disorders, or on anticonvulsant medication for epilepsy should not take this substance. The drug interaction might produce seizures. Some patients have been shown to have an upset stomach, severe abdominal cramping, and vomiting issues connected to taking this drug.[35]
Phenylephrine is pregnancy category C. Due to the lack of studies done in animals and in humans, it is not known whether there is harm to the fetus. Phenylephrine should only be given to pregnant women who have a clear need.[35]
Extended use may cause rhinitis medicamentosa, a condition of rebound nasal congestion.[36]
Interactions
The increase in blood pressure effect of phenylephrine may be increased by drugs such as monoamine oxidase inhibitors, tricyclic antidepressants, and hydrocortisone. Patients taking these medications may need a lower dose of phenylephrine to achieve a similar increase in blood pressure.
Drugs that may decrease the effects of phenylephrine may include calcium channel blockers, ACE inhibitors and benzodiazepines. Patients taking these medications may need a higher dose of phenylephrine to achieve a comparable increase in blood pressure.[37]
Pharmacology
Pharmacodynamics
Phenylephrine is a sympathomimetic drug, which means that it mimics the actions of epinephrine (commonly known as adrenaline) or norepinephrine. Phenylephrine selectively binds to α1-adrenergic receptors which causes venous and arterial vasoconstriction.[31][38]
Whereas pseudoephedrine causes both vasoconstriction and increase of mucociliary clearance through its non-specific adrenergic activity, phenylephrine's selective α1-adrenergic receptor agonism causes vasoconstriction alone, creating a difference in their methods of action.
Pharmacokinetics
Oral phenylephrine is extensively metabolized by monoamine oxidase,[1] an enzyme that is present on the mitochondrial membrane of cells throughout the body.[39] Compared to oral pseudoephedrine, phenylephrine has a reduced and variable bioavailability; only up to 38%.[1][40]
References
- 1 2 3 "Phenylephrine (DB00388)". DrugBank. Retrieved 4 April 2015.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Phenylephrine Monograph for Professionals". Drugs.com. AHFS. 2 March 2022. Retrieved 9 May 2022.
However, efficacy of oral phenylephrine for this use [as a decongestant] has been questioned.
- 1 2 Richards E, Lopez MJ, Maani CV (2023). "Phenylephrine". StatPearls. Treasure Island, Florida: StatPearls Publishing. PMID 30521222. Retrieved 27 April 2023.
- 1 2 3 Joint Formulary Committee (2018). BNF 76 : September 2018 - March 2019. London: British Medical Association, Royal Pharmaceutical Society of Great Britain. pp. 188–189. ISBN 9780857113382. OCLC 1021215075.
- ↑ U.S. Patent 1,932,347, application 1928, expired 1950
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 541. ISBN 9783527607495.
- ↑ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ↑ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
- ↑ "Max Strength Decongestant Tablets" (PDF). www.mhra.gov.uk. p. 10. Archived from the original (PDF) on 19 August 2019. Retrieved 10 January 2019.
- ↑ Hatton RC, Hendeles L (March 2022). "Why Is Oral Phenylephrine on the Market After Compelling Evidence of Its Ineffectiveness as a Decongestant?". Ann Pharmacother. 56 (11): 1275–1278. doi:10.1177/10600280221081526. PMID 35337187. S2CID 247712448.
- ↑ Lowe D (March 2022). "The Uselessness of Phenylephrine". Science (blog).
- ↑ Christensen J (12 September 2023). "Popular OTC medicines for colds and allergies don't work, FDA panel says". CNN. Retrieved 8 October 2023.
- ↑ Presley B, Bianchi B, Coleman J, Diamond F, McNally G (July 2018). "Efficiency of extraction and conversion of pseudoephedrine to methamphetamine from tamper-resistant and non-tamper-resistant formulations". Journal of Pharmaceutical and Biomedical Analysis. 156: 16–22. doi:10.1016/j.jpba.2018.04.016. PMID 29684907. S2CID 13660478.
- 1 2 Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Yao R, Staudinger H, et al. (February 2009). "A placebo-controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber". Annals of Allergy, Asthma & Immunology. 102 (2): 116–20. doi:10.1016/S1081-1206(10)60240-2. PMID 19230461.
Phenylephrine was not significantly different from placebo in the primary end point.
- 1 2 Day JH, Briscoe MP, Ratz JD, Danzig M, Yao R (April 2009). "Efficacy of loratadine-montelukast on nasal congestion in patients with seasonal allergic rhinitis in an environmental exposure unit". Annals of Allergy, Asthma & Immunology. 102 (4): 328–38. doi:10.1016/S1081-1206(10)60339-0. PMID 19441605.
There were no statistically significant differences between phenylephrine and placebo for any measures.
- ↑ Hendeles L, Hatton RC (July 2006). "Oral phenylephrine: an ineffective replacement for pseudoephedrine?". The Journal of Allergy and Clinical Immunology. 118 (1): 279–80. doi:10.1016/j.jaci.2006.03.002. PMID 16815167.
- ↑ Hatton RC, Winterstein AG, McKelvey RP, Shuster J, Hendeles L (March 2007). "Efficacy and safety of oral phenylephrine: systematic review and meta-analysis". The Annals of Pharmacotherapy. 41 (3): 381–90. doi:10.1345/aph.1H679. PMID 17264159. S2CID 25627664. Archived from the original (abstract) on 27 February 2007.(published online Jan 2007)
- ↑ Kollar C, Schneider H, Waksman J, Krusinska E (June 2007). "Meta-analysis of the efficacy of a single dose of phenylephrine 10 mg compared with placebo in adults with acute nasal congestion due to the common cold". Clinical Therapeutics. 29 (6): 1057–70. doi:10.1016/j.clinthera.2007.05.021. PMID 17692721.
- ↑ Desjardins PJ, Berlin RG (October 2007). "Efficacy of phenylephrine". British Journal of Clinical Pharmacology. 64 (4): 555–6, author reply 557. doi:10.1111/j.1365-2125.2007.02935.x. PMC 2048561. PMID 17610531.
- ↑ Hillenmeyer K (30 January 2007). "All stuffed up: Reformulated cold medicines might not be able to do the job". Sarasota Herald-Tribune. Archived from the original on 1 March 2007. Retrieved 25 April 2023.
- ↑ "FDA clarifies results of recent AC meeting on oral phenylephrine". U.S. Food and Drug Administration (FDA). 14 September 2023. Retrieved 14 September 2023.
- ↑ Christensen J (12 September 2023). "Popular OTC medicines for colds and allergies don't work, FDA panel says". CNN. Retrieved 12 September 2023.
- ↑ Constantino AK (12 September 2023). "Decongestant found in many cold, allergy medicines doesn't actually work, FDA advisors say". CNBC. Retrieved 12 September 2023.
- ↑ "Hemorrhoids". Mayo Clinic.
- ↑ "Phenylephrine rectal". WebMD. Retrieved 4 April 2015.
- ↑ "Preparation H – cocoa butter and phenylephrine hydrochloride suppository". DailyMed. U.S. National Institutes of Health. Retrieved 4 April 2015.
- ↑ "Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% — Sterile" (PDF). Akorn. Archived from the original (PDF) on 3 March 2016.
- ↑ Bizrah M, Corbett MC (March 2019). "Intracameral Phenylephrine to Arrest Intraoperative Intraocular Bleeding: A New Technique". Ophthalmology and Therapy. 8 (1): 137–141. doi:10.1007/s40123-019-0165-y. PMC 6393249. PMID 30771215.
- ↑ Kanfer I, Dowse R, Vuma V (1993). "Pharmacokinetics of oral decongestants". Pharmacotherapy. 13 (6 Pt 2): 116S–128S, discussion 143S–146S. doi:10.1002/j.1875-9114.1993.tb02780.x. PMID 7507589. S2CID 23528004.
- ↑ Cooper BE (2008). "Review and update on inotropes and vasopressors". AACN Advanced Critical Care. 19 (1): 5–13, quiz 14–5. doi:10.1097/01.AACN.0000310743.32298.1d. PMID 18418098. S2CID 39192378.
- 1 2 "Phenylephrine hydrochloride injection". DailyMed. U.S. National Institutes of Health. Retrieved 4 April 2015.
- ↑ "Phenylephrine (Rx)". Medscape. Retrieved 4 April 2015.
- ↑ Stavert B, McGuinness MB, Harper CA, Guymer RH, Finger RP (June 2015). "Cardiovascular Adverse Effects of Phenylephrine Eyedrops: A Systematic Review and Meta-analysis". JAMA Ophthalmology. 133 (6): 647–52. doi:10.1001/jamaophthalmol.2015.0325. PMID 25789577.
- ↑ Shen H (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 3. ISBN 978-1-59541-101-3.
- 1 2 "Phenylephrine Hydrochloride injection, for intravenous use" (PDF). U.S. Food and Drug Administration.
- ↑ "Neo-Synephrine Nasal Spray Drug Information, Professional". drugs.com. Archived from the original on 11 May 2015. Retrieved 4 April 2015.
- ↑ "Vazculep Package Insert" (PDF). U.S. Food and Drug Administration.
- ↑ Richards E, Lopez MJ, Maani CV (11 July 2022). "Phenylephrine". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. PMID 30521222.
- ↑ Shih JC, Chen K (August 2004). "Regulation of MAO-A and MAO-B gene expression". Current Medicinal Chemistry. 11 (15): 1995–2005. doi:10.2174/0929867043364757. PMID 15279563.
- ↑ "Recommendation on phenylephrine". Medsafe. 23 May 2013 [25 November 2004]. Retrieved 25 April 2023.