 | |
| Clinical data | |
|---|---|
| Other names | WIN-17665; Propyltestosterone; 17α-Propyltestosterone; 17α-Propylandrost-4-en-17β-ol-3-one |
| Routes of administration | Topical |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.638 |
| Chemical and physical data | |
| Formula | C22H34O2 |
| Molar mass | 330.512 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
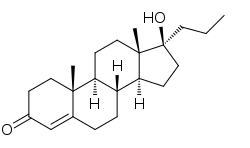
Topterone (INN, USAN) (developmental code name WIN-17665), also known as 17α-propyltestosterone (or simply propyltestosterone) or as 17α-propylandrost-4-en-17β-ol-3-one, is a steroidal antiandrogen that was first reported in 1978 and was developed for topical administration but, due to poor effectiveness, was never marketed.[1][2][3][4][5][6]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1–. ISBN 978-1-4757-2085-3.
- ↑ Ferrari RA, Chakrabarty K, Beyler AL, Wiland J (November 1978). "Suppression of sebaceous gland development in laboratory animals by 17alpha-propyltestosterone". The Journal of Investigative Dermatology. 71 (5): 320–323. doi:10.1111/1523-1747.ep12529809. PMID 712108.
- ↑ Rasmusson GH (1986). "Chapter 18. Chemical Control of Androgen Action". Annual Reports in Medicinal Chemistry. Vol. 21. Academic Press. pp. 179–188 (183). doi:10.1016/S0065-7743(08)61128-8. ISBN 978-0-08-058365-5.
- ↑ Ferrari RA, Chakrabarty K, Creange JE, Beyler AL, Potts OG, Schane HP (April 1980). "Endocrine profile of topterone, a topical antiandrogen, in three species of laboratory animals". Methods and Findings in Experimental and Clinical Pharmacology. 2 (2): 65–69. PMID 7339330.
- ↑ Chakrabarty K, Ferrari RA, Dessingue OC, Beyler AL, Schane HP (January 1980). "Mechanism of action of 17 alpha-propyltestosterone in inhibiting hamster flank organ development". The Journal of Investigative Dermatology. 74 (1): 5–8. doi:10.1111/1523-1747.ep12514560. PMID 7351494.
- ↑ Marsden JR, Shuster S (6 December 2012). "The Treatment of Acne". Pharmacology of the Skin II: Methods, Absorption, Metabolism and Toxicity, Drugs and Diseases. Springer Science & Business Media. pp. 490–. ISBN 978-3-642-74054-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.