 | |
| Clinical data | |
|---|---|
| Other names | 15β-Hydroxy-CPA; 15β-OH-CPA; 6-Chloro-15β,17α-dihydroxy-1α,2α-methylenepregna-4,6-diene-3,20-dione 17α-acetate; 6-Chloro-1,2α-methylene-15β,17α-dihydroxy-δ6-progesterone 17α-acetate |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H29ClO5 |
| Molar mass | 432.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
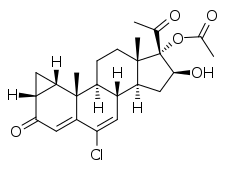
15β-Hydroxycyproterone acetate (15β-OH-CPA) is a steroidal antiandrogen and the major metabolite of cyproterone acetate (CPA).[1][2][3] It is formed from CPA in the liver by hydroxylation via the cytochrome P450 enzyme CYP3A4.[1][2][3] During therapy with CPA, 15β-OH-CPA circulates at concentrations that are approximately twice those of CPA.[4] 15β-OH-CPA has similar or even greater antiandrogen activity compared to CPA.[5] However, it has only about one-tenth of the activity of CPA as a progestogen.[6] 15β-OH-CPA also shows some glucocorticoid activity, similarly to CPA and unesterified cyproterone.[7][8]
See also
References
- 1 2 Saleh FM (11 February 2009). "Pharmacological Treatment of Paraphilic Sex Offenders". In Saleh FM (ed.). Sex Offenders: Identification, Risk Assessment, Treatment, and Legal Issues. Oxford University Press, USA. pp. 197–. ISBN 978-0-19-517704-6.
- 1 2 Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przegla̜d Menopauzalny = Menopause Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- 1 2 Boarder M, Newby D, Navti P (25 March 2010). "Pharmacogical Treatment of Cancer". Pharmacology for Pharmacy and the Health Sciences: A Patient-centred Approach. OUP Oxford. pp. 632–. ISBN 978-0-19-955982-4.
- ↑ Frith RG, Phillipou G (February 1985). "15-Hydroxycyproterone acetate and cyproterone acetate levels in plasma and urine". Journal of Chromatography. 338 (1): 179–186. doi:10.1016/0378-4347(85)80082-7. PMID 3160716.
- ↑ Neumann F (22 October 2013). "Pharmacological basis for clinical use of antiandrogens". In James VH, Pasqualini JR (eds.). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier. pp. 398–. ISBN 978-1-4831-9067-9.
- ↑ Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ↑ Bhargava AS, Kapp JF, Poggel HA, Heinick J, Nieuweboer B, Günzel P (1981). "Effect of cyproterone acetate and its metabolites on the adrenal function in man, rhesus monkey and rat". Arzneimittel-Forschung. 31 (6): 1005–1009. PMID 6266428.
- ↑ Excerpta medica. Section 30. Pharmacology and toxicology. 1982.
In addition, cyprosterone acetate and its main metabolite, 15β-hydroxy acetate, as well as the newly isolated free alcohol of the main metabolite were characterized for their corticosteroid-like activity in rats. Cyproterone acetate treatment of male hypersexual subjects and female rhesus monkeys did not reveal any signs of adrenal suppression. Cyproterone acetate and its metabolites gave no indication of any appreciable antiinflammatory effect in the adjuvant edema test in rats. However, there was a general increase in the level of blood glucose and liver glycogen as well as a reduction in body weight and organ weight (spleen, thymus and adrenal) in rats, in which 15β-hydroxy cyproterone was slightly more active with the exception of adrenal weight reduction, which was almost equal in all three compounds tested. It can also be concluded that adult man and rhesus monkey are much less sensitive, if at all so, to some corticosteroid-like activities of cyproterone acetate and its main metabolites than the rat.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.