 | |
| Names | |
|---|---|
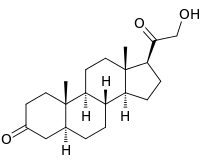
| IUPAC name
21-Hydroxy-5α-pregnane-3,20-dione | |
| Systematic IUPAC name
(1S,3aS,3bR,5aS,9aS,9bS,11aS)-1-(Hydroxyacetyl)-9a,11a-dimethylhexadecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
5α-Dihydro-11-deoxycorticosterone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H32O3 | |
| Molar mass | 332.484 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5α-Dihydrodeoxycorticosterone (abbreviated as DHDOC), also known as 21-hydroxy-5α-pregnan-20-one, is an endogenous progestogen and neurosteroid.[1] It is synthesized from the adrenal hormone deoxycorticosterone (DOC) by the enzyme 5α-reductase type I.[1] DHDOC is an agonist of the progesterone receptor, as well as a positive allosteric modulator of the GABAA receptor, and is known to have anticonvulsant effects.[1][2]
Chemistry
See also: List of neurosteroids
See also
- Tetrahydrodeoxycorticosterone (THDOC)
- 5α-Dihydroprogesterone (DHP)
- Hydroxydione
References
- 1 2 3 Reddy DS, Rogawski MA (May 2002). "Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility". J. Neurosci. 22 (9): 3795–805. doi:10.1523/JNEUROSCI.22-09-03795.2002. PMC 6758375. PMID 11978855.
- ↑ Edwards HE, Vimal S, Burnham WM (December 2005). "The acute anticonvulsant effects of deoxycorticosterone in developing rats: role of metabolites and mineralocorticoid-receptor responses". Epilepsia. 46 (12): 1888–97. doi:10.1111/j.1528-1167.2005.00295.x. PMID 16393154. S2CID 26030656.
| PRTooltip Progesterone receptor |
| ||||||
|---|---|---|---|---|---|---|---|
| mPRTooltip Membrane progesterone receptor (PAQRTooltip Progestin and adipoQ receptor) |
| ||||||
| |||||||
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.