 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
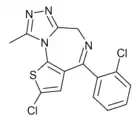
| Formula | C15H10Cl2N4S |
| Molar mass | 349.23 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 205 °C (401 °F) |
| |
| |
Clotizolam (Ro11-1465) is a thienotriazolodiazepine derivative first invented in the 1970s, which in more recent years has been sold as a designer drug. As with other related thienotriazolodiazepines, it produces sedative, anxiolytic, anticonvulsant and muscle relaxant effects,[1] and also acts as an inhibitor of platelet-activating factor (PAF).[2]
See also
References
- ↑ US 4155913, Hellerbach J, Zeller P, Binder D, Hromatka O, "Thienotriazolodiazepine derivatives", issued 22 May 1979, assigned to Hoffmann La Roche Inc.
- ↑ Tahara T, Mikashima H, Terasawa M, Maruyama Y (May 1987). "PAF antagonistic activity of some thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepines". Chemical & Pharmaceutical Bulletin. 35 (5): 2119–21. doi:10.1248/cpb.35.2119. PMID 3664818. S2CID 27564672.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.