 | |
| Clinical data | |
|---|---|
| Other names | THG; The Clear; 17α-Ethyl-18-methyl-δ9,11-19-nortestosterone; 17α-Ethyl-18-methylestra-4,9,11-trien-17β-ol-3-one |
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
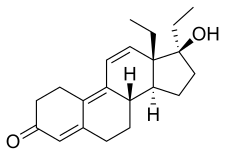
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tetrahydrogestrinone (THG), known by the nickname The Clear, is a synthetic and orally active anabolic–androgenic steroid (AAS) which was never marketed for medical use.[1][2] It was developed by Patrick Arnold and was used by a number of high-profile athletes such as Marion Jones, Barry Bonds, and Dwain Chambers.
Non-medical uses
THG was developed completely in secret by Arnold as a designer drug, on the basis that doping testers would be unlikely to detect a totally new compound. Arnold developed a chemical similar to two obscure steroids marketed by BALCO, norbolethone and desoxymethyltestosterone, which had been reported in scientific literature but never entered mass production, and the banned anabolic steroids trenbolone and gestrinone, the latter of which was used to synthesize it.[3]
In 2003, whistleblower Trevor Graham passed a spent syringe containing a small amount of the drug to the United States Anti-Doping Agency. This was then transferred to the research group of pharmacologist Don Catlin, who identified the drug using mass spectrometry techniques and gave it its present name.[4][5]
THG has never been fully tested for safety and has never entered legitimate medical use, although some studies have been made of its properties.[6] A synthesis was devised to ensure a source of material for comparison and it was scheduled by the Food and Drug Administration (FDA) in 2005.[7][1] Concerns have also been raised about its potential use in animals such as in horse-racing.
Side effects
Side effects from prolonged use are likely to include infertility in both men and women, as well as other steroid side effects such as acne and hirsutism.[8] Unlike most other anabolic steroids, THG also binds with high affinity to the glucocorticoid receptor, and while this effect may cause additional weight loss, it is also likely to cause additional side effects such as immunosuppression that are not seen with most other steroids.[9]
Pharmacology
Pharmacodynamics
THG is a highly potent agonist of the androgen and progesterone receptors,[10] around 10 times more potent than the comparison drugs nandrolone or trenbolone, but with no estrogenic activity. It has been found to bind to the androgen receptor with similar affinity to dihydrotestosterone and produces growth of muscle tissue.[11] According to Patrick Arnold, due to the drug's potency, he never had to supply significant quantities to BALCO, because "just a couple of drops under the tongue" were a sufficient dose.[2]
When THG reaches the nucleus of a cell, it binds to the androgen receptor at the ligand-binding pocket. Here it changes the expression of a variety of genes, turning on several anabolic and androgenic functions.[12] It is the ligand's structure which determines the number of interactions that can take place with the human androgen receptor ligand-binding domain. Even minor modifications in the ligand's structure have a great impact on the strength of the interactions this ligand has with the androgen receptor. THG, possessing a high affinity, establishes more van der Waals contacts with the receptor than with many other steroids. It is this higher affinity and specific geometry of THG which makes these interactions with the androgen receptor so strong, resulting in THG's potency.[13]
Chemistry
THG, also known as 17α-ethyl-18-methyl-δ9,11-19-nortestosterone or as 17α-ethyl-18-methylestra-4,9,11-trien-17β-ol-3-one, is a synthetic estrane steroid and a 17α-alkylated derivative of nandrolone (19-nortestosterone). It is a modification of gestrinone (17α-ethynyl-18-methyl-19-nor-δ9,11-testosterone) in which the ethynyl group has been hydrogenated into an ethyl group, thereby converting the steroid from a norethisterone (17α-ethynyl-19-nortestosterone) derivative with weak AR activity into a norethandrolone (17α-ethyl-19-nortestosterone) derivative with powerful AR activity. THG is closely related to RU-2309 (the 17α-methyl variant), trenbolone (δ9,11-19-nortestosterone), metribolone (17α-methyl-δ9,11-19-nortestosterone), and norboletone (17α-ethyl-18-methyl-19-nortestosterone).
History
For a time, THG was considered the drug of choice for safe and "invisible" world record breaking in athletics, being used by several high-profile gold medal winners such as the sprinter Marion Jones, who resigned from her athletic career in 2007 after admitting to using THG prior to the 2000 Sydney Olympics, where she had won three gold medals.[14] It has also been used by formerly banned British athlete Dwain Chambers, Major League Baseball left fielder Barry Bonds, and Major League Baseball first baseman Jason Giambi.[15]
THG was developed by Patrick Arnold for the Bay Area Laboratory Co-operative (BALCO), which claimed to be a nutritional supplement company.[16] The company manufactured the drug through palladium-charcoal catalyzed hydrogenation from gestrinone, a substance used in gynecology for treatment of endometriosis (Australian Medicines handbook 2011).
In 2003, U.S. sprint coach Trevor Graham delivered a syringe containing traces of THG to the United States Anti-Doping Agency (USADA). This helped Don Catlin, MD, the founder and then-director of the UCLA Olympic Analytical Lab, to identify and develop a test for THG, the second reported designer anabolic steroid.[17]
References
- 1 2 US 2006045847, "Method for determination of anabolic activity"
- 1 2 "Video". CNN. 2006-10-09. Archived from the original on 2013-01-02. Retrieved 2010-05-25.
- ↑ Cotton S. "Molecule of the month: THG". University of Bristol. Archived from the original on 7 March 2016. Retrieved 3 September 2016.
- ↑ Montoya G. "Dr. Don Catlin on Anti-Doping in Boxing". Max Boxing. Archived from the original on 17 September 2016. Retrieved 3 September 2016.
- ↑ Catlin DH, Sekera MH, Ahrens BD, Starcevic B, Chang YC, Hatton CK (30 June 2004). "Tetrahydrogestrinone: discovery, synthesis, and detection in urine". Rapid Communications in Mass Spectrometry. 18 (12): 1245–049. Bibcode:2004RCMS...18.1245C. doi:10.1002/rcm.1495. PMID 15174177.
- ↑ Ghigo E, Lanfranco F, Strasburger CJ (28 October 2010). Hormone Use and Abuse by Athletes. Springer Science & Business Media. p. 80. ISBN 978-1-4419-7014-5.
- ↑ "Oct.2003 FDA statement on THG". fda.gov. Archived from the original on 13 May 2009. Retrieved 7 May 2018.
- ↑ Death AK, McGrath KC, Kazlauskas R, Handelsman DJ (May 2004). "Tetrahydrogestrinone is a potent androgen and progestin". The Journal of Clinical Endocrinology and Metabolism. 89 (5): 2498–500. doi:10.1210/jc.2004-0033. PMID 15126583.
- ↑ Friedel A, Geyer H, Kamber M, Laudenbach-Leschowsky U, Schänzer W, Thevis M, et al. (June 2006). "Tetrahydrogestrinone is a potent but unselective binding steroid and affects glucocorticoid signalling in the liver". Toxicology Letters. 164 (1): 16–23. doi:10.1016/j.toxlet.2005.11.006. PMID 16356667.
- ↑ Labrie F, Luu-The V, Calvo E, Martel C, Cloutier J, Gauthier S, et al. (February 2005). "Tetrahydrogestrinone induces a genomic signature typical of a potent anabolic steroid". The Journal of Endocrinology. 184 (2): 427–33. doi:10.1677/joe.1.05997. PMID 15684350.
- ↑ Jasuja R, Catlin DH, Miller A, Chang YC, Herbst KL, Starcevic B, et al. (October 2005). "Tetrahydrogestrinone is an androgenic steroid that stimulates androgen receptor-mediated, myogenic differentiation in C3H10T1/2 multipotent mesenchymal cells and promotes muscle accretion in orchidectomized male rats". Endocrinology. 146 (10): 4472–8. doi:10.1210/en.2005-0448. PMID 15976054.
- ↑ Kicman AT (June 2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ↑ Thevis M, Schänzer W (2005). "Mass Spectrometry in Doping Control Analysis". Current Organic Chemistry. 9 (9): 825–848. doi:10.2174/1385272054038318.
- ↑ "Jones pleads guilty in drug case". BBC News. 2007-10-06. Retrieved 2010-05-25.
- ↑ Fainaru-Wada M, Lance W (December 2, 2004). "Giambi admitted taking steroids". San Francisco Chronicle. Retrieved May 25, 2007.
- ↑ "Chemist Who Create "The Clear" Sentenced". United States Department of Justice. 4 August 2006. Archived from the original on 2006-10-14. Retrieved 2007-10-08.
- ↑ Steeg JL (28 February 2007). "Catlin has made a career out of busting juicers". USA Today. Archived from the original on 2009-02-26. Retrieved 2009-03-31.
External links
- The identity of the whistle-blowing coach
- "This Is Very Clever Chemistry" from The Washington Post, December 4, 2004