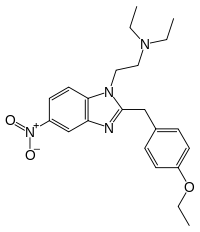
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.827 |
| Chemical and physical data | |
| Formula | C22H28N4O3 |
| Molar mass | 396.491 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etonitazene, also known as EA-4941 or CS-4640,[2] is a benzimidazole opioid, first reported in 1957,[3] that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.[4]
Because it is characterized by a strong dependency potential and a tendency to produce profound respiratory depression, it is not used in humans. It is, however, useful in animal models for addiction studies, particularly those requiring the animals to drink or ingest the agent, because it is not as bitter as opiate salts like morphine sulfate.
Synthesis
Etonitazene and its related opioid agonist benzimidazoles were discovered in the late 1950s,[5][6][7][8][9][10] by a team of Swiss researchers working at the pharmaceutical firm CIBA (now Novartis). One of the first compounds investigated by the Swiss team was 1-(β-diethylaminoethyl)-2-benzylbenzimidazole, which was found to possess 10% of the analgesic activity of morphine when tested in rodent bioassays. This finding encouraged the group to begin a comprehensive systematic study of 2-benzylbenzimidazoles and to establish the structure-activity relationship of this new family of analgesics. Two general synthetic methods were developed for the preparation of these compounds.
The first method involved the condensation of o-phenylenediamine with para-ethoxy-phenylacetonitrile to form a 2-benzylbenzimidazole. The benzimidazole is then alkylated with the desired 1-chloro-2-dialkylaminoethane, forming the final product. This particular procedure was most useful for the preparation of benzimidazoles that lacked substituents on the benzene rings. A diagram of this method is displayed below.[11]
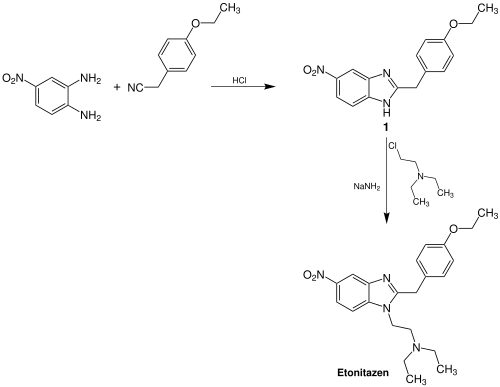
The most versatile synthesis[6] developed by the Swiss team first involved alkylation of 2,4-dinitrochlorobenzene with 1-amino-2-diethylaminoethane to form N-(β-Diethylaminoethyl)-2,4-dinitroaniline [aka: N'-(2,4-Dinitrophenyl)-N,N-diethyl-ethane-1,2-diamine]. The 2-nitro substituent o n the 2,4-dinitroaniline compound is then selectively reduced to the corresponding primary amine by utilizing ammonium sulfide as the reducing agent. The ammonium sulfide can be formed in situ by the addition of concentrated aqueous ammonium hydroxide followed by saturation of the solution with hydrogen sulfide gas. The intermediate formed by the selective reduction of the 2-nitro substituent, 2-(β-Diethylaminoethylamino)-5-nitroaniline, is then reacted with the hydrochloride salt of the imino ethyl ether of 4-ethoxyphenylacetonitrile (aka: p-ethoxybenzyl cyanide). The imino ether, 2-(4-Ethoxyphenyl)-acetimidic acid ethyl ester hydrochloride, is prepared by dissolving the 4-substituted benzyl cyanide in a mixture of anhydrous ethanol and chloroform and then saturating this solution with dry hydrogen chloride gas. The reaction between the 2-(β-dialkylaminoalkylamine)-5-nitroaniline and the HCl salt of the imino ethyl ether results in the formation of etonitazene. This procedure is particularly useful in the preparation of the 4-, 5-, 6-, and 7-nitrobenzimidazoles. Varying the choice of the substituted phenylacetic acid imino ether affords compounds with a diversity of substituents on the benzene ring at the 2- position. A diagram of this particular synthesis as it applies to the preparation of etonitazene is shown below.[5]

A particularly novel, high-yielding synthesis of etonitazene was developed by FI Carroll and MC Coleman in the mid-1970s[12] The authors were tasked with the preparation of large quantities of etonitazene, but found the conventional synthesis to be inadequate. The problem with the conventional synthesis was the lability of the imino ether reactant, 2-(4-Ethoxyphenyl)-acetimidic acid ethyl ester (prepared by reacting 4-ethoxyphenylacetonitrile with ethanolic HCl). The imino ether necessitated the use of anhydrous reaction conditions and was inconvenient to prepare in large quantities. This led the authors to experiment with the use of a coupling reagent, EEDQ (N-Ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline), in order to promote the condensation of 2-(2-diethylaminoethylamino)-5-nitroaniline with 4-ethoxyphenylacetic acid. The authors discovered that when this condensation was performed in the presence of 2 or more molar equivalents of EEDQ (added portionwise in 3 steps) in THF at 50 °C for 192 hours (8 days), a near quantitative yield (100%) of etonitazene was obtained. In addition to the impressive improvement in yield over the conventional procedure, the work up procedure was greatly simplified since quinoline, carbon dioxide, and ethanol were the only by-products formed. A diagram of this procedure is shown below.
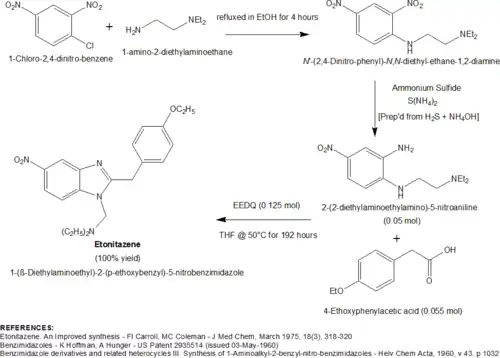
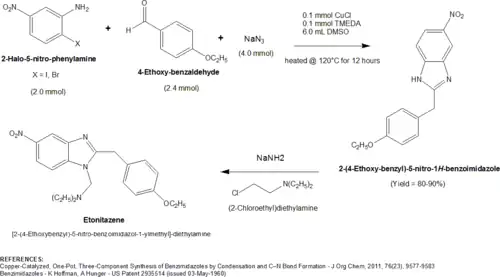
A 2011 publication[13] [J. Org. Chem., 2011, 76(23), 9577-9583] from a South Korean team outlined a novel, one-pot synthesis for substituted and unsubstituted 2-benzyl-benzimidazoles that can be easily adapted to the preparation of etonitazene. The three component synthesis of the direct etonitazene precursor, 2-(4-Ethoxybenzyl)-5-nitro-1H-benzoimidazole, consists of a 2-Bromo- or 2-Iodo-5-nitro-phenylamine (1.0 molar equivalent), a 4-substituted benzaldehyde (1.2 equiv), and sodium azide (2 equiv). The 2-Halo-5-nitro-phenylamine requires a bromo or iodo group for optimal activity. 2-Chloro-phenylamines are completely unreactive. In addition to these three components, the reaction was optimized in the presence of 0.05 molar equivalents (5 mol%) of a catalyst, copper(I) chloride, and 5 mol% of ligand, TMEDA (tetramethylethylenediamine). After heating these components at 120 °C for 12 hours in DMSO, the direct etonitazene precursor, 2-(4-Ethoxybenzyl)-5-nitro-1H-benzoimidazole, was formed in an approx 80-90% yield. The secondary amine nitrogen of 2-(4-Ethoxybenzyl)-5-nitro-1H-benzoimidazole was then alkylated with (2-Chloroethyl)diethylamine to form etonitazene.[11] A diagram of this synthesis is shown below.
Analogs
A number of analogues are known, with the only other well-known compound to come out of the original 1950s research being clonitazene, which is much weaker than etonitazene (around 3x morphine). More recently since around late 2018 a number of designer analogues have started to appear on illicit markets around the world, with the most prominent compounds being metonitazene, isotonitazene and etazene, though others have continued to appear.[14][15]
Of these analogues, only etonitazene and clonitazene are explicitly listed under UN conventions and so are controlled throughout the world. The rest would only be illegal in countries such as the US, Australia and New Zealand that have laws equivalent to the Federal Analog Act. In the United States Etonitazene is a Schedule I narcotic controlled substance with a DEA ACSCN of 9624 and a 25 gram (⅞ oz) manufacturing quota as of 2022.
Illicit production
Illicit production and sale of etonitazene has been limited. Identified on the Moscow illegal drug market in 1998, it was primarily smoked in laced cigarettes.[16] A chemist at Morton Thiokol synthesized the compound for his own use.[17] The drug was produced in Russia in 1996 and sold as 'Chinese Dwarf'. The drug resulted in an unconfirmed number of deaths due to its uncertain potency.[18] It appears to have a steep dose-response curve, and unpredictable pharmacokinetics especially when injected, in a similar manner to some other potent opioids such as dextromoramide, which may cause etonitazene to be especially hazardous when compared to opioids of similar potency such as fentanyl.[19]
See also
- Etazen (desnitroetonitazene)
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ King JW, Cleveland JP, Lennox WJ (November 1991). Williams JD, Reeves PJ (eds.). Synthesis and Bioactivity of 2-(Alpha-Hydroxy-Para-Alkoxybenzyl) and 2-Alkoxy Arylamino Analogs of Etonitazene (CS - 4640). Proceedings of the U.S. Army Chemical Research, Development and Engineering Center Scientific Conference on Chemical Defense Research. Aberdeen Proving Ground, Maryland: Edgewood Research, Development and Engineering Center, Aberdeen Proving Ground, MD.
- ↑ US patent 2935514, Hoffmann K, Hunger A, Kebrle J, Rossi A, "Benzimidazoles", published 1957-09-19, issued 1960-05-03, assigned to Ciba Pharmaceutical Products Inc., Summit, NJ.
- ↑ Wikler A, Martin WR, Pescor FT, Eades CG (October 1963). "Factors Regulating Oral Consumption of an Opioid (Etonitazene) by Morphine-Addicted Rats". Psychopharmacologia. 5 (1): 55–76. doi:10.1007/BF00405575. PMID 14082382. S2CID 38073529.
- 1 2 Hunger A, Kebrle J, Rossi A, Hoffmann K (October 1957). "[Synthesis of analgesically active benzimidazole derivatives with basic substitutions]" [Synthesis of analgesically active benzimidazole derivatives with basic substitutions]. Experientia. 13 (10): 400–401. doi:10.1007/BF02161116. PMID 13473817. S2CID 32179439.
- 1 2 Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen. IV. Die Kondensation von o-Phenylendiamin mit α-Aryl- und γ-Aryl-acetessigester" [Benzimidazole derivatives and related heterocycles IV. The condensation of o-phenylenediamine with α-aryl and γ-aryl-acetoacetate]. Helvetica Chimica Acta (in German). 43 (4): 1046–1056. doi:10.1002/hlca.19600430413.
- ↑ Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen V. Die Kondensation von o-Phenylendiamin mit aliphatischen und alicyclischen β-Ketoestern" [Benzimidazole derivatives and related heterocycles V. The condensation of o-phenylenediamine with aliphatic and alicyclic β-keto esters]. Helvetica Chimica Acta (in German). 43 (5): 1298–1313. doi:10.1002/hlca.19600430515.
- ↑ Hunger A, Kebrle J, Rossi A, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen VI. Synthese von Phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden" [Benzimidazole derivatives and related Heterocycles VI. Synthesis of phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-acetic acid esters and amides]. Helvetica Chimica Acta (in German). 43 (6): 1727–1733. doi:10.1002/hlca.19600430634.
- ↑ Hunger A, Kebrle J, Rossi A, Hoffmann K (1961). "Benzimidazol-Derivate und verwandte Heterocyclen VII. Synthese neuer 2-Amino-benzimidazole" [Benzimidazole Derivatives and related Heterocycles VII. Synthesis of new 2-amino-benzimidazole]. Helvetica Chimica Acta (in German). 44 (5): 1273–1282. doi:10.1002/hlca.19610440513.
- ↑ Gross F, Turrian H (October 1957). "[Benzimidazole derivatives with strong analgesic effects]" [Benzimidazole derivatives with strong analgesic effects]. Experientia. 13 (10): 401–403. doi:10.1007/BF02161117. PMID 13473818. S2CID 6824038.
- 1 2 Hunger A, Kebrle J, Rossi A, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen. II. Synthese von 1-Aminoalkyl-2-benzyl-benzimidazolen" [Benzimidazole derivatives and related heterocycles II. Synthesis of 1-aminoalkyl-2-benzyl-benzimidazoles]. Helvetica Chimica Acta (in German). 43 (3): 800–809. doi:10.1002/hlca.19600430323.
- ↑ Carroll FI, Coleman MC (March 1975). "Etonitazene. An improved synthesis". Journal of Medicinal Chemistry. 18 (3): 318–320. doi:10.1021/jm00237a024. PMID 237125.
- ↑ Kim Y, Kumar MR, Park N, Heo Y, Lee S (December 2011). "Copper-catalyzed, one-pot, three-component synthesis of benzimidazoles by condensation and C-N bond formation". The Journal of Organic Chemistry. 76 (23): 9577–9583. doi:10.1021/jo2019416. PMID 22034860.
- ↑ Blanckaert P, Cannaert A, Van Uytfanghe K, Hulpia F, Deconinck E, Van Calenbergh S, Stove C (April 2020). "Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene". Drug Testing and Analysis. 12 (4): 422–430. doi:10.1002/dta.2738. PMID 31743619. S2CID 208187034.
- ↑ Vandeputte M, Van Uytfanghe K, Layle N, Germaine DS, Iula D, Stove C (12 November 2020). "Synthesis, chemical characterization, and µ-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids". Authorea. doi:10.22541/au.160520665.59016513/v1. S2CID 234646245.
- ↑ Sorokin VI, Ponkratov KV, Drozdov MA (1999). "Etonitazene Encountered in Moscow". Microgram. 32 (9): 239–244.
- ↑ Reavy P (3 June 2003). "Chemist charged in drug case". Deseret News. Salt Lake City. Archived from the original on March 7, 2014.
- ↑ "Этонитазен (etonitazene) - описание препарата" [Etonitazene (etonitazene) - a description of the drug] (in Russian). Social Services LLC. Archived from the original on 23 March 2019.
- ↑ Kishioka S, Ko MC, Woods JH (May 2000). "Diltiazem enhances the analgesic but not the respiratory depressant effects of morphine in rhesus monkeys". European Journal of Pharmacology. 397 (1): 85–92. doi:10.1016/s0014-2999(00)00248-x. PMID 10844102.