 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Delatestryl, Xyosted, others |
| Other names | TE; Testosterone heptanoate; Testosterone 17β-heptanoate; NSC-17591 |
| Routes of administration | Intramuscular injection, subcutaneous injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: very low Intramuscular: high |
| Metabolism | Liver |
| Elimination half-life | Intramuscular: 4–5 days[2] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.005.686 |
| Chemical and physical data | |
| Formula | C26H40O3 |
| Molar mass | 400.603 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Testosterone enanthate is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels in men.[3][4][5] It is also used in hormone therapy for transgender men.[6] It is given by injection into muscle or subcutaneously usually once every one to four weeks.[5][7][2]
Side effects of testosterone enanthate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[5] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[8][5] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy.[5] Testosterone enanthate is a testosterone ester and a long-lasting prodrug of testosterone in the body.[7][3][4] Because of this, it is considered to be a natural and bioidentical form of testosterone.[9]
Testosterone enanthate was introduced for medical use in 1954.[10][4] Along with testosterone cypionate, testosterone undecanoate, and testosterone propionate, it is one of the most widely used testosterone esters.[8][4][5] In addition to its medical use, testosterone enanthate is used to improve physique and performance.[5] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[5]
Medical uses
Testosterone enanthate is used primarily in androgen replacement therapy.[4][11] It is the most widely used form of testosterone in androgen replacement therapy.[4] The medication is specifically approved, in the United States, for the treatment of hypogonadism in men, delayed puberty in boys, and breast cancer in women.[12] It is also used in masculinizing hormone therapy for transgender men.[6]
Side effects
Side effects of testosterone enanthate include virilization among others.[5] Approximately 10 percent of testosterone enanthate will be converted to dihydrotestosterone in normal men.[13] Dihydrotestosterone (DHT) can promote masculine characteristics in both males and females. These masculine characteristics include: clitoral hypertrophy, androgenic alopecia, growth of body hair and deepening of the vocal cords. Dihydrotestosterone also plays an important role in male sexual function and may also be a contributing factor of ischemic priapism in males as shown in a study conducted on the use of finasteride to treat ischemic priapism in males. Testosterone enanthate can also lead to an increase in igf-1 and igf-bp.[14][15] Testosterone enanthate can also be converted to estradiol by aromatase,[16] which may lead to gynecomastia in males. Aromatase inhibitors can help to prevent the estrogenic activity of testosterone enanthate in the body.[16]
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Testosterone enanthate is a prodrug of testosterone and is an androgen and anabolic–androgenic steroid (AAS). That is, it is an agonist of the androgen receptor (AR).
Pharmacokinetics
Testosterone enanthate has an elimination half-life of 4.5 days and a mean residence time of 8.5 days when used as a depot intramuscular injection.[2] It requires frequent administration of approximately once per week, and large fluctuations in testosterone levels result with it, with levels initially being elevated and supraphysiological.[2]
Chemistry
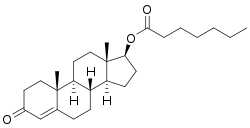
Testosterone enanthate, or testosterone 17β-heptanoate, is a synthetic androstane steroid and a derivative of testosterone.[17][18] It is an androgen ester; specifically, it is the C17β enanthate (heptanoate) ester of testosterone.[17][18]
History
Testosterone enanthate was described as early as 1952[19] and was first introduced for medical use in the United States in 1954 under the brand name Delatestryl.[10][4]
Society and culture
Generic names
Testosterone enanthate is the generic name of the drug and its USAN and BAN.[17][18][20][21] It has also referred to as testosterone heptanoate.[17][18][20][21]
Brand names
Testosterone enanthate is marketed primarily under the brand name Delatestryl.[17][18][20][21]
It is or has been marketed under a variety of other brand names as well, including, among others:[17][18][20][21][22]
- Andro LA
- Andropository
- Cypionat
- Cypoprime
- Depandro
- Durathate
- Everone
- Testocyp
- Testostroval
- Testrin
- Testro LA
- Xyosted
- pharmaqo labs
Availability
Testosterone enanthate is available in the United States and widely elsewhere throughout the world.[23][18][21] Testosterone enanthate (testosterone heptanoate) is often available in concentrations of 200 mg per milliliter of fluid.[24]
Legal status
Testosterone enanthate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[25][26]
Research
As of October 2017, an auto-injection formulation of testosterone enanthate was in preregistration for the treatment of hypogonadism in the United States.[27]
Xyosted
On October 1, 2018, the U.S. Food and Drug Administration (FDA) announced the approval of Xyosted. Xyosted, a product of Antares Pharma, Inc., is a single-use disposable auto-injector that dispenses testosterone enanthate. Xyosted is the first FDA-approved subcutaneous testosterone enanthate product for testosterone replacement therapy in adult males.[28]
References
- ↑ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- 1 2 3 4 Luetjens CM, Wistuba J, Weinbauer G, Nieschlag E (2007). "The Leydig Cell as a Target for Male Contraception". The Leydig Cell in Health and Disease. Contemporary Endocrinology. Humana Press. pp. 415–442. doi:10.1007/978-1-59745-453-7_29. ISBN 978-1-58829-754-9.
- 1 2 Nieschlag E, Behre HM, Nieschlag S (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 315–. ISBN 978-1-107-01290-5.
- 1 2 3 4 5 6 7 Nieschlag E, Behre HM, Nieschlag S (13 January 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 442–. ISBN 978-3-540-78355-8.
- 1 2 3 4 5 6 7 8 9 Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 208–211. ISBN 978-0-9828280-1-4.
- 1 2 Irwig MS (April 2017). "Testosterone therapy for transgender men". The Lancet. Diabetes & Endocrinology. 5 (4): 301–311. doi:10.1016/S2213-8587(16)00036-X. PMID 27084565.
- 1 2 Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185, 1187. ISBN 978-0-7817-1750-2.
- 1 2 Kicman AT (June 2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ↑ Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". The Journal of Clinical Endocrinology and Metabolism. 101 (4): 1318–1343. doi:10.1210/jc.2016-1271. PMID 27032319.
- 1 2 "Testosterone Enanthate". p. 35t. in William Andrew Publishing (2007). "T". Pharmaceutical Manufacturing Encyclopedia. pp. 1t–242t. doi:10.1016/B978-0-8155-1526-5.50024-6. ISBN 978-0-8155-1526-5.
- ↑ "Testosterone Enanthate raw powder (CAS 315-37-7) ≥98% | AASraw". aasraw. Retrieved 2022-11-20.
- ↑ "DELATESTRYL Package Insert" (PDF). Indevus Pharmaceuticals, Inc.
- ↑ "DHT (dihydrotestosterone): What is DHT's role in baldness?". 28 July 2017.
- ↑ Ashton WS, Degnan BM, Daniel A, Francis GL (1995). "Testosterone increases insulin-like growth factor-1 and insulin-like growth factor-binding protein". Annals of Clinical and Laboratory Science. 25 (5): 381–388. PMID 7486812.
- ↑ Hoeh MP, Levine LA (March 2015). "Management of Recurrent Ischemic Priapism 2014: A Complex Condition with Devastating Consequences". Sexual Medicine Reviews. 3 (1): 24–35. doi:10.1002/smrj.37. PMID 27784569. S2CID 24028084.
- 1 2 Ishikawa T, Glidewell-Kenney C, Jameson JL (February 2006). "Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5 alpha-reductase inhibitor". The Journal of Steroid Biochemistry and Molecular Biology. 98 (2–3): 133–138. doi:10.1016/j.jsbmb.2005.09.004. PMID 16386416. S2CID 25849126.
- 1 2 3 4 5 6 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–642. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1002–1004. ISBN 978-3-88763-075-1.
- ↑ Junkmann K (1952). "Über protrahiert wirksame Androgene" [Over protracted effective androgens]. Festschrift zum 75. Geburtstag. Springer. pp. 85–92. doi:10.1007/978-3-642-49902-9_11. ISBN 978-3-642-49610-3.
- 1 2 3 4 Morton IK, Hall JM (6 December 2012). "Testosterone". Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. p. 270. ISBN 978-94-011-4439-1.
- 1 2 3 4 5 "Testosterone". Drugs.com. October 1, 2018. Retrieved December 5, 2018.
- ↑ "Testosterone cypionate profile and most popular brands in USA". Anabolic Steroids Ratings and Reviews - downsizefitness.com. Retrieved 2020-09-06.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 17 December 2016.
- ↑ "Testosterone enanthate". Drugbank.
- ↑ Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- ↑ Lilley LL, Snyder JS, Collins SR (5 August 2016). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. pp. 50–. ISBN 978-1-77172-066-3.
- ↑ "Testosterone enanthate auto-injection - Antares Pharma". AdisInsight. February 5, 2018. Retrieved December 5, 2018.
- ↑ "Antares Receives Fda Approval of Xyostedtm (Testosterone Enanthate) Injection for Testosterone Replacement Therapy in Adult Males" (PDF).